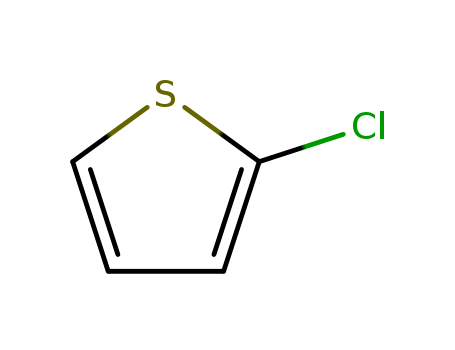
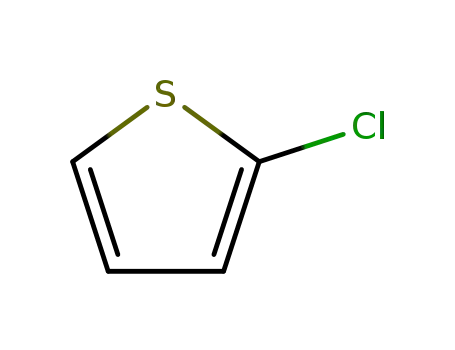
Manufacturer supply top purity 2-Chlorothiophene 96-43-5 with GMP standards
- Molecular Formula:C4H3ClS
- Molecular Weight:118.587
- Appearance/Colour:Colorless to light yellow liquid
- Vapor Pressure:13mmHg at 25°C
- Melting Point:-71.9 °C
- Refractive Index:1.547 - 1.549
- Boiling Point:128.3 °C at 760 mmHg
- Flash Point:22.8 °C
- PSA:28.24000
- Density:1.305 g/cm3
- LogP:2.40150
2-Chlorothiophene(Cas 96-43-5) Usage
|
General Description
|
The photodissociation dynamics of 2-chlorothiophene was studied using resonance enhanced multiphoton ionization (REMPI) time-of-flight (TOF) technique. |
|
Purification Methods
|
Purify it by fractional distillation at atmospheric pressure or by gas chromatography. [Conde et al. Synthesis 412 1976, Beilstein 17/1 V 303.] |
InChI:InChI=1/C4H3ClS/c5-4-2-1-3-6-4/h1-3H
96-43-5 Relevant articles
Activator free, expeditious and eco-friendly chlorination of activated arenes by N-chloro-N-(phenylsulfonyl)benzene sulfonamide (NCBSI)
Misal, Balu,Palav, Amey,Ganwir, Prerna,Chaturbhuj, Ganesh
supporting information, (2021/01/04)
N-Chloro-N-(phenylsulfonyl)benzene sulfo...
Dehydroxyalkylative halogenation of C(aryl)-C bonds of aryl alcohols
Liu, Mingyang,Zhang, Zhanrong,Liu, Huizhen,Wu, Tianbin,Han, Buxing
supporting information, p. 7120 - 7123 (2020/07/14)
We herein report Cu mediated side-direct...
Highly-chemoselective step-down reduction of carboxylic acids to aromatic hydrocarbons: Via palladium catalysis
Liu, Chengwei,Qin, Zhi-Xin,Ji, Chong-Lei,Hong, Xin,Szostak, Michal
, p. 5736 - 5742 (2019/06/18)
Aryl carboxylic acids are among the most...
Synthesis method of 2-thiopheneacetic acid
-
Paragraph 0028; 0051; 0052, (2017/01/26)
The invention provides a synthesis metho...
96-43-5 Process route
-
-
188290-36-0,8014-23-1,25233-34-5
thiophene
-
-
598-49-2
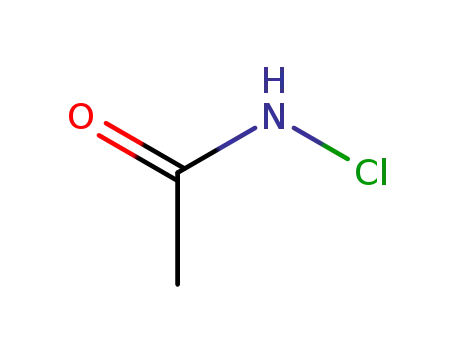
N-chloroacetamide
-
-
96-43-5
2-thienyl chloride
-
-
3172-52-9,173777-89-4
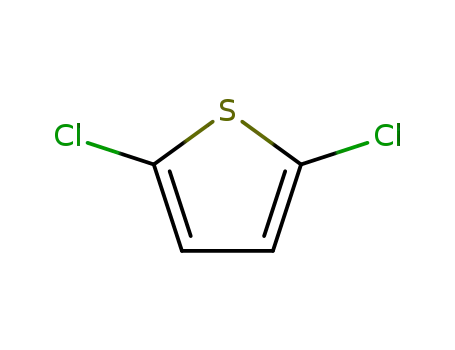
2,5-diclorothiophene
Conditions
| Conditions |
Yield |
|
at 60 ℃;
|
|
-
-
188290-36-0,8014-23-1,25233-34-5
thiophene
-
-
96-43-5
2-thienyl chloride
-
-
3172-52-9,173777-89-4
2,5-diclorothiophene
Conditions
| Conditions |
Yield |
|
With
ammonium nitrate; N-chloro-succinimide;
In
acetonitrile;
for 1h;
|
59%
10%
|
|
With
hydrogenchloride; sodium peroxide;
In
toluene; benzene;
at 28 - 30 ℃;
for 0.5h;
Product distribution;
var. reagents; var. temperatures, var. reaction times;
|
67 % Chromat.
11 % Chromat.
|
|
With
chlorine; potassium carbonate;
|
|
96-43-5 Upstream products
96-43-5 Downstream products
-
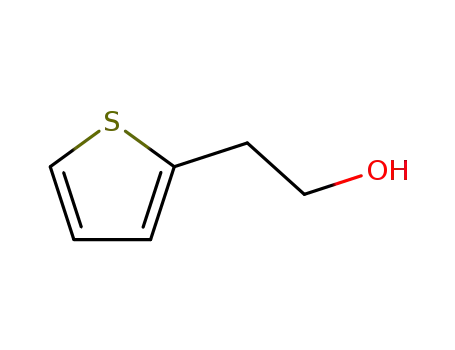
5402-55-1
2-thiophenethanol
-
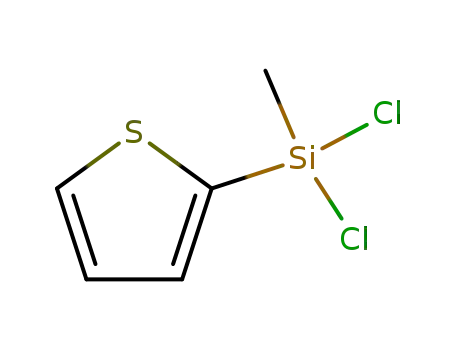
18243-72-6
2-(methyldichlorosilyl)thiophene
-
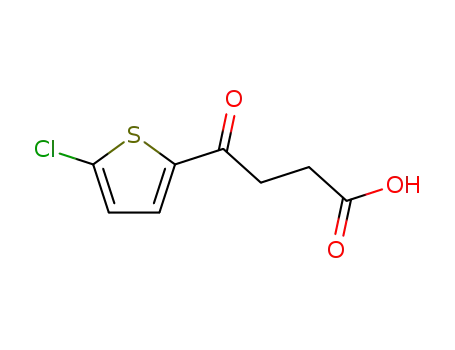
70685-06-2
3-(5-chlorothienoyl)propionic acid
-
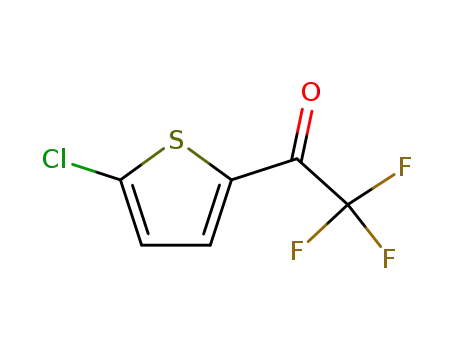
771-83-5
1-(5-chloro-[2]thienyl)-2,2,2-trifluoro-ethanone
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego