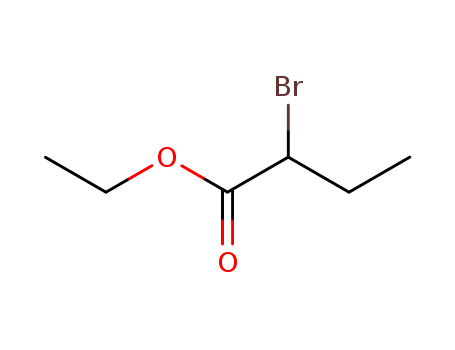
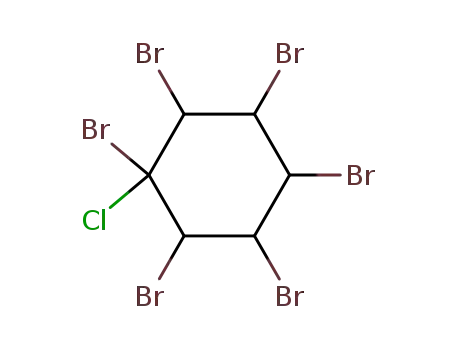
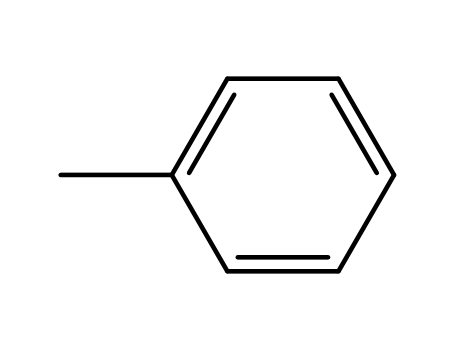
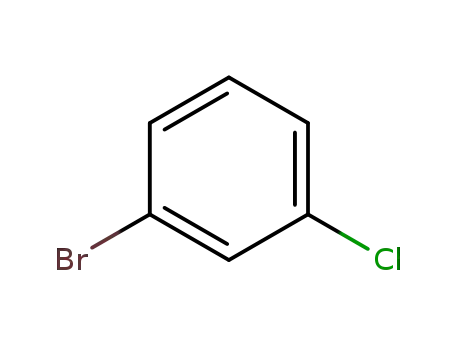
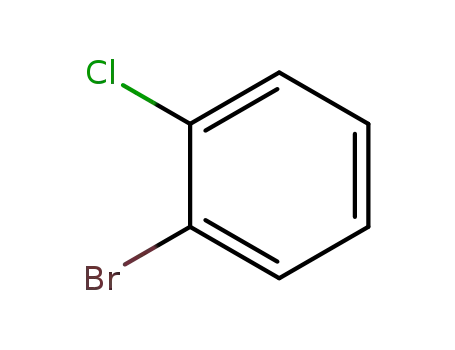
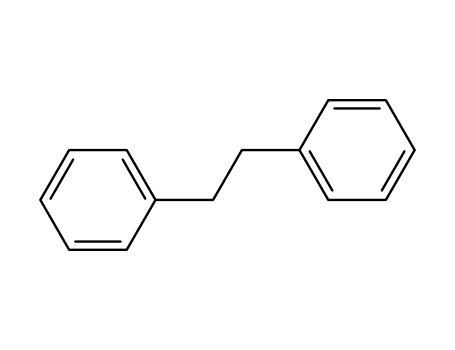
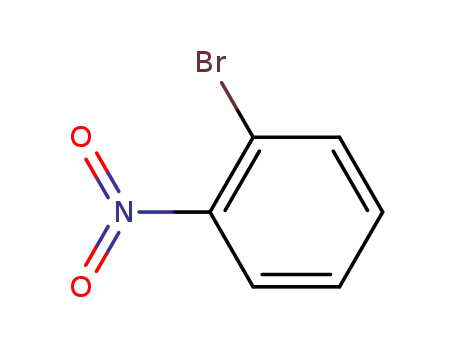
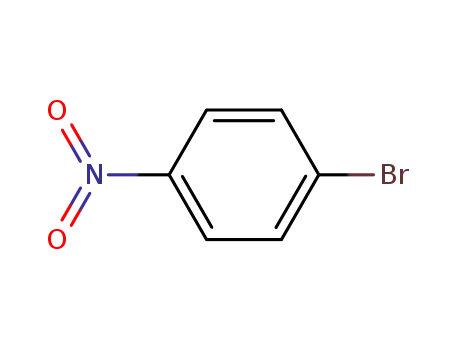
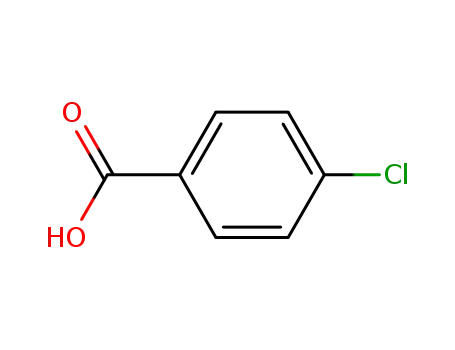
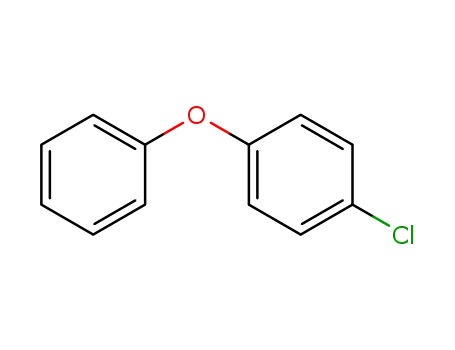
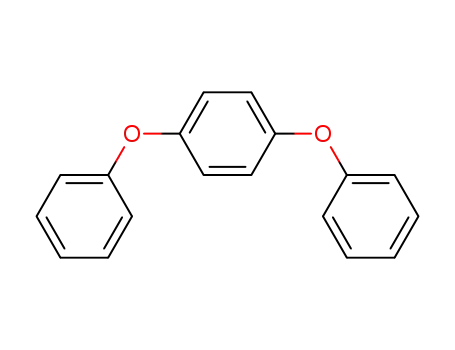
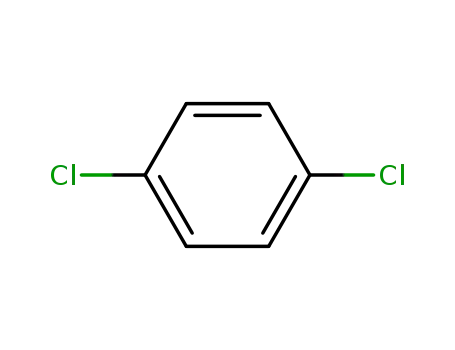
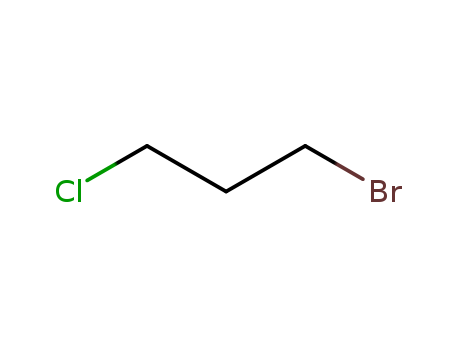
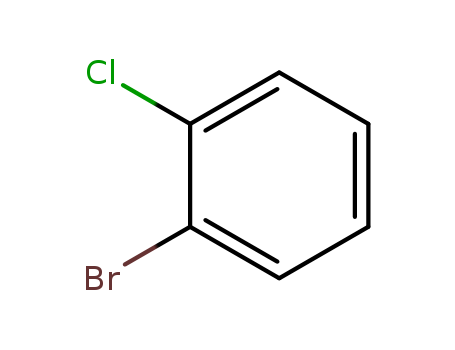
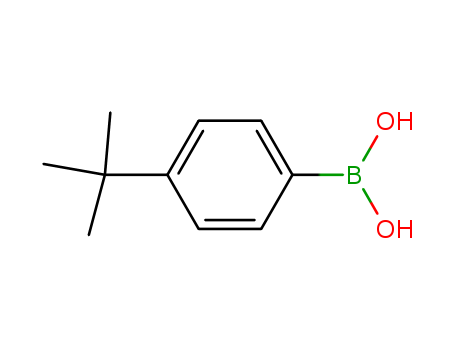
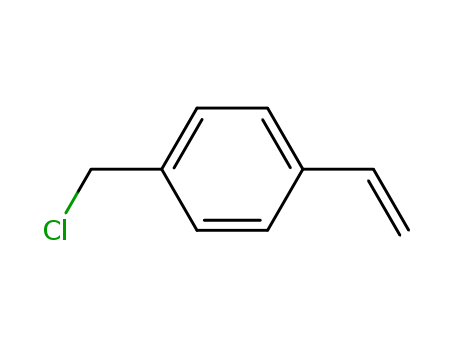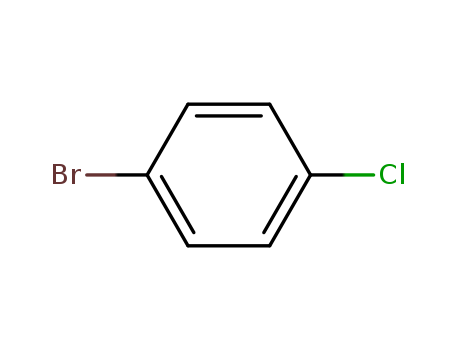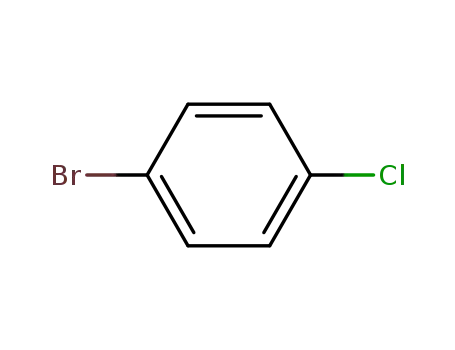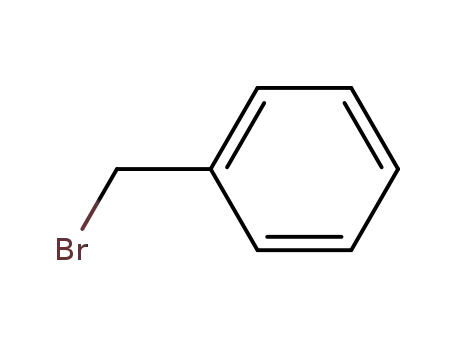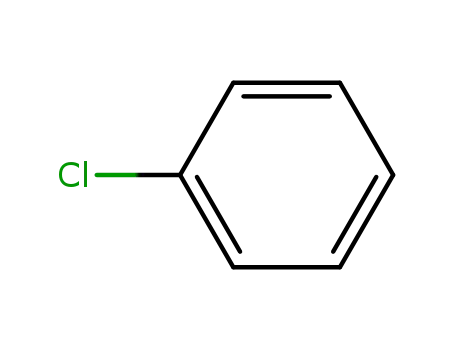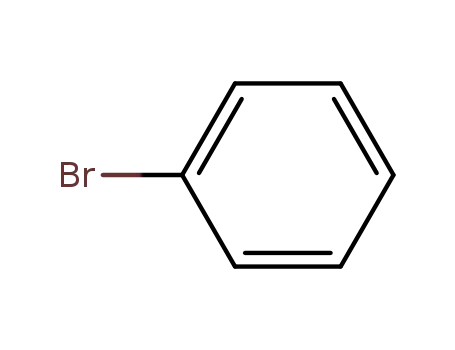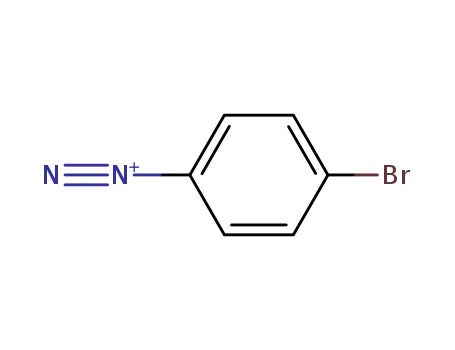-
Derbyshire,Waters
, p. 573,576 (1950)
-
Mild chlorination of aromatic compounds with tin(IV) chloride and lead tetraacetate
Muathen, Hussni A.
, p. 8863 - 8866 (1996)
SnCl4/Pb(OAc)4 acts as a safe source of ...
Eco-compatible zeolite-catalysed continuous halogenation of aromatics
Losch,Kolb,Astafan,Daou,Pinard,Pale,Louis
, p. 4714 - 4724 (2016)
A completely eco-compatible halogenation...
Bromination of Deactivated Aromatics Using BrF3 without a Catalyst
Rozen, Shlomo,Lerman, Ori
, p. 239 - 240 (1993)
-
Highly para-Selective Mono-Chlorination of Aromatic Compounds Under Mild Conditions by t-Butyl Hypochlorite in the Presence of Zeolites
Smith, Keith,Butters, Michael,Nay, Barry
, p. 1157 - 1158 (1985)
t-Butyl hypochlorite supported on H(1+),...
-
Kovacic,Sparks
, p. 5740 (1960)
-
-
Gokel et al.
, p. 1633 (1977)
-
Decarboxylative Ipso Halogenation of Mercury(II) Pyridinecarboxylates. Facile Formation of 3-Iodo- and 3-Bromopyridines
Uemura, Sakae,Tanaka, Sakuya,Okano, Masaya,Hamana, Masatomo
, p. 3297 - 3301 (1983)
Treatment of mercury(II) nicotinate with...
Poly-N-bromosulfonamide-melamine as a novel brominating reagent for regioselective ipso-bromination of arylboronic acids
Alavinia, Sedigheh,Ghorbani-Vaghei, Ramin
, p. 1269 - 1276 (2021/08/27)
A practical synthetic method for the syn...
The graphite-catalyzed: ipso -functionalization of arylboronic acids in an aqueous medium: metal-free access to phenols, anilines, nitroarenes, and haloarenes
Badgoti, Ranveer Singh,Dandia, Anshu,Parewa, Vijay,Rathore, Kuldeep S.,Saini, Pratibha,Sharma, Ruchi
, p. 18040 - 18049 (2021/05/29)
An efficient, metal-free, and sustainabl...
Orthogonal Stability and Reactivity of Aryl Germanes Enables Rapid and Selective (Multi)Halogenations
Deckers, Kristina,Fricke, Christoph,Schoenebeck, Franziska
supporting information, p. 18717 - 18722 (2020/08/25)
While halogenation is of key importance ...
Metal- and base-free synthesis of aryl bromides from arylhydrazines
Phuc Tran, Dat,Nomoto, Akihiro,Mita, Soichiro,Dong, Chun-ping,Kodama, Shintaro,Mizuno, Takumi,Ogawa, Akiya
supporting information, (2020/05/08)
An efficient method was developed to syn...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego