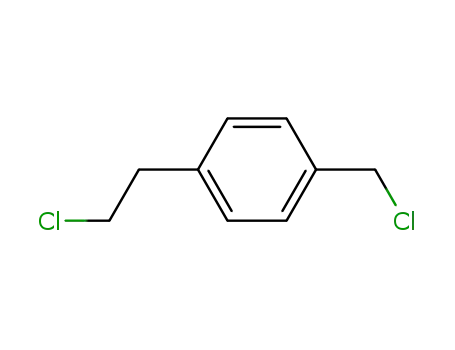
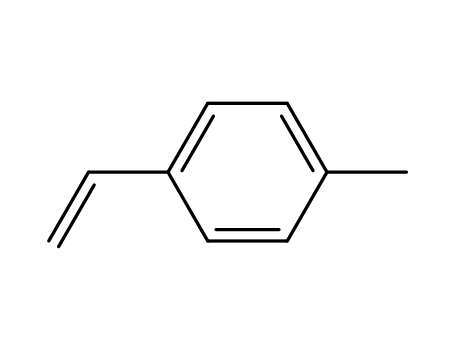
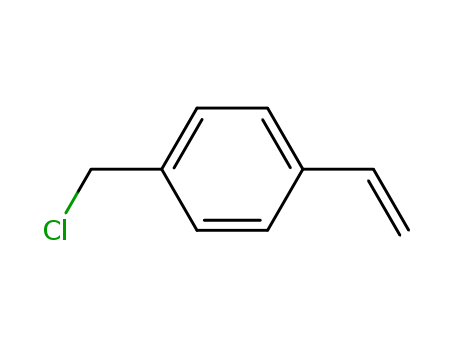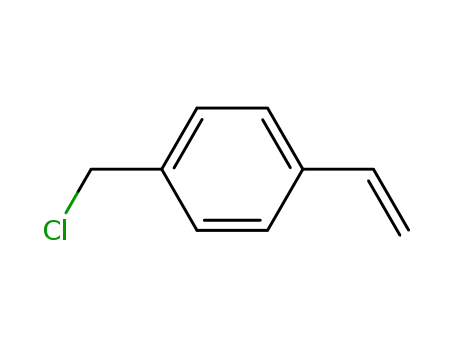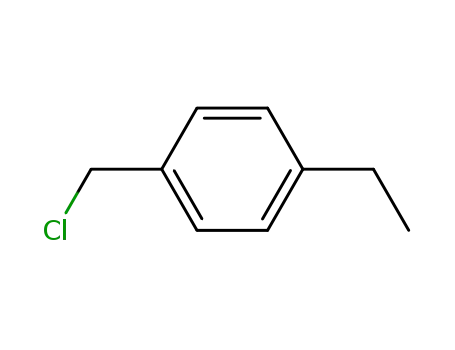Quality Manufacturer Supply High Purity 99% 4-Vinylbenzyl chloride 1592-20-7 with Reasonable Price
- Molecular Formula:C9H9Cl
- Molecular Weight:152.623
- Appearance/Colour:clear yellow liquid
- Vapor Pressure:1 mm Hg ( 56.1 °C)
- Refractive Index:n20/D 1.572(lit.)
- Boiling Point:228.9 °C at 760 mmHg
- Flash Point:90.4 °C
- PSA:0.00000
- Density:1.066 g/cm3
- LogP:3.06840
4-Vinylbenzyl chloride(Cas 1592-20-7) Usage
|
Purification Methods
|
Purify 4-vinylbenzyl chloride by dissolving it in Et2O, washing it with 0.5% of aqueous NaOH, separating, drying the organic layer (Na2SO4), evaporating and distilling the residual oil under N2 in vacuo. Add 0.05% of 4-tert-butylcatechol as stabilizer. It is lachrymatory. [Nishikubo et al. Tetrahedron Lett 22 3872 1981, Tanimoto et al. Synth Commun 4 193 1974, Beilstein 6 IV 3818.] |
InChI:InChI=1/C9H9Cl/c1-2-8-3-5-9(7-10)6-4-8/h2-6H,1,7H2
1592-20-7 Relevant articles
FACILE SYNTHESIS OF p-CHLOROMETHYLATED STYRENE BY ELIMINATION REACTION OF p-(2-BROMOETHYL)BENZYLCHLORIDE USING POTASSIUM HYDROXIDE AS A BASE UNDER PHASE TRANSFER CATALYSIS
Nishikubo, Tadatomi,Iizawa, Takashi,Kobayashi, Kazuo,Okawara, Makoto
, p. 3873 - 3874 (1981)
Phase transfer catalyzed elimination rea...
Preparation method of P-chloromethyl styrene
-
Paragraph 0076-0090, (2021/05/01)
The invention relates to the field of or...
Synthesis method of p-chloromethyl styrene
-
Paragraph 0047-0048, (2019/11/25)
The invention relates to a synthesis met...
Highly selective halogenation of unactivated C(sp3)-H with NaX under co-catalysis of visible light and Ag@AgX
Liu, Shouxin,Zhang, Qi,Tian, Xia,Fan, Shiming,Huang, Jing,Whiting, Andrew
, p. 4729 - 4737 (2018/10/23)
The direct selective halogenation of una...
Structurally Defined Molecular Hypervalent Iodine Catalysts for Intermolecular Enantioselective Reactions
Haubenreisser, Stefan,W?ste, Thorsten H.,Martnez, Claudio,Ishihara, Kazuaki,Muiz, Kilian
supporting information, p. 413 - 417 (2016/01/25)
Molecular structures of the most promine...
1592-20-7 Process route
-
-
35793-35-2
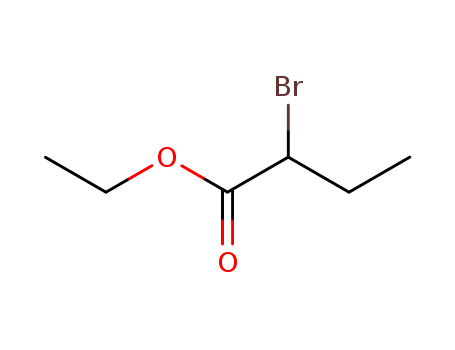
p-chloromethyl-α-bromoethylbenzene
-
-
1592-20-7,29296-32-0
4-Vinylbenzyl chloride
Conditions
| Conditions |
Yield |
|
With
18-crown-6 ether; potassium hydroxide;
In
toluene;
at 40 ℃;
for 4h;
Reagent/catalyst;
Temperature;
Solvent;
|
95%
|
-
-
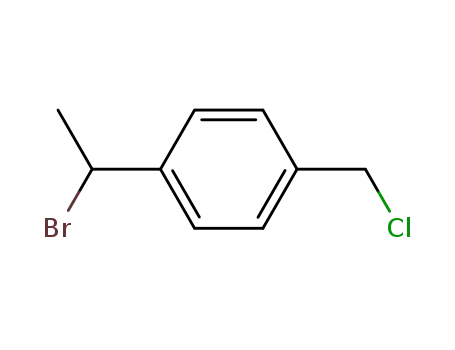
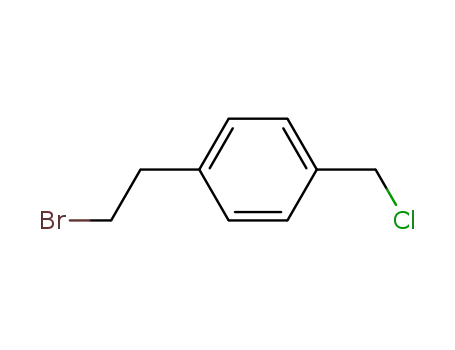
53459-40-8
1-(2-chloroethyl)-4-(chloromethyl)benzene
-
-
1592-20-7,29296-32-0
4-Vinylbenzyl chloride
Conditions
| Conditions |
Yield |
|
With
4-tert-Butylcatechol; sodium t-butanolate;
In
tetrahydrofuran;
at 30 ℃;
for 1h;
Reagent/catalyst;
Solvent;
Inert atmosphere;
|
83%
|
1592-20-7 Upstream products
-
1467-05-6
4-ethylbenzylchloride
-
24249-98-7
p-chloromethyl-2-bromoethylbenzene
-
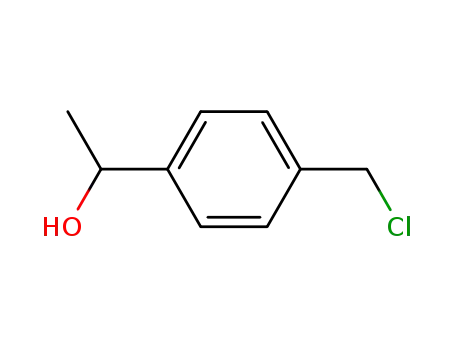
76297-21-7
1-(4-chloromethylphenyl)ethanol
-
622-97-9
1-ethenyl-4-methylbenzene
1592-20-7 Downstream products
-
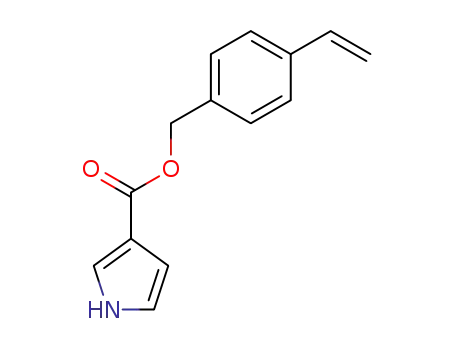
134226-18-9
p-vinylbenzyl 1-H-pyrrole-3-carboxylate
-
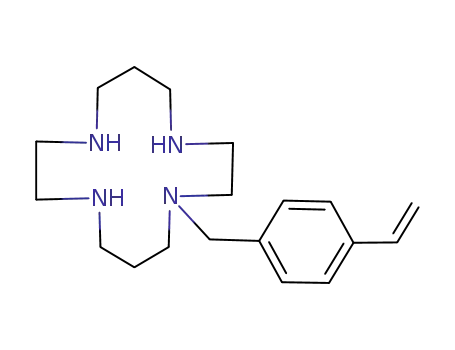
121383-67-3
1-(4-vinylbenzyl)cyclam
-
93253-93-1
bis(n-octadecylmethyl)(p-vinylbenzyl)-ammonium chloride
-
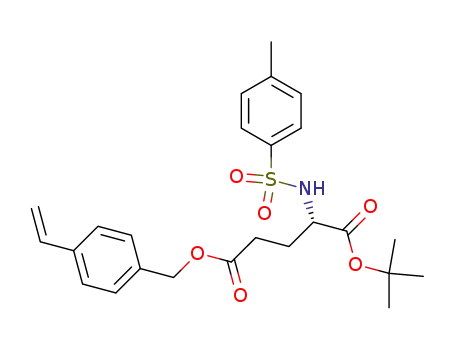
159046-71-6
(S)-2-(Toluene-4-sulfonylamino)-pentanedioic acid 1-tert-butyl ester 5-(4-vinyl-benzyl) ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego