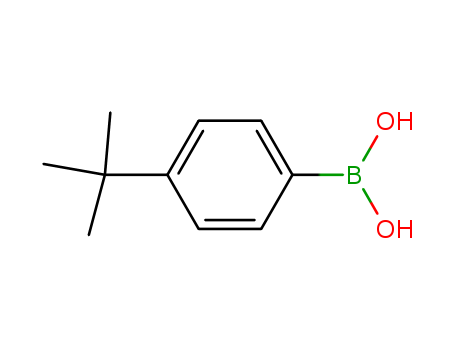
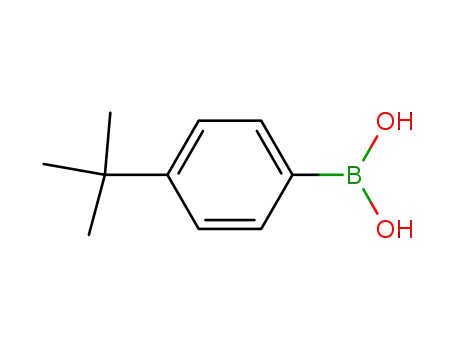
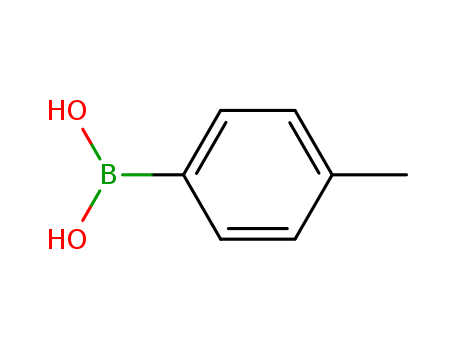
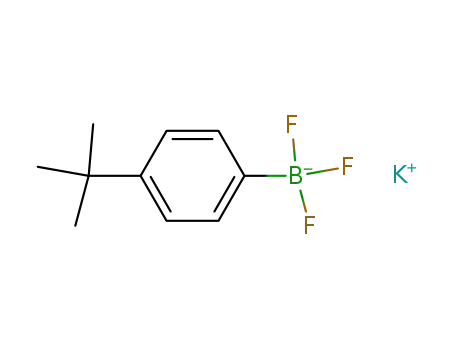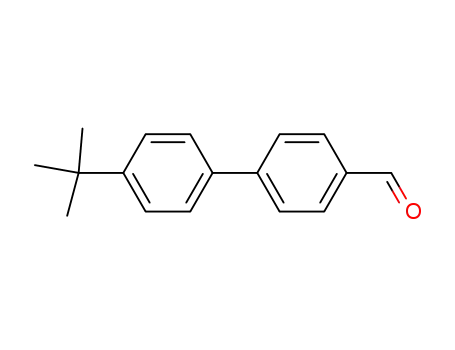Manufacturer supply high quality 4-tert-Butylphenylboronic acid 123324-71-0 with GMP standards
- Molecular Formula:C10H15BO2
- Molecular Weight:178.039
- Appearance/Colour:white to off-white crystalline powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:193-199 ºC
- Refractive Index:1.59
- Boiling Point:296.7 ºC at 760 mmHg
- PKA:8.79±0.10(Predicted)
- Flash Point:133.2 ºC
- PSA:40.46000
- Density:1.02 g/cm2
- LogP:0.66390
4-tert-Butylphenylboronic acid(Cas 123324-71-0) Usage
4-tert-Butylphenylboronic acid is a biochemical reagent used in various scientific research fields, including organic synthesis, pharmaceutical development, and material science.
InChI:InChI=1/C7H7BO4/c9-8(10)5-1-2-6-7(3-5)12-4-11-6/h1-3,9-10H,4H2
123324-71-0 Relevant articles
Synthesis of t-butylated diphenylanthracene derivatives as blue host materials for OLED applications
Balaganesan, Banumathy,Shen, Wen-Jian,Chen, Chin H.
, p. 5747 - 5750 (2003)
This paper describes the cost-effective ...
New C2v- and chiral C2-symmetric olefin polymerization catalysts based on nickel(II) and palladium(II) diimine complexes bearing 2,6-diphenyl aniline moieties: Synthesis, structural characterization, and first insight into polymerization properties
Schmid, Markus,Eberhardt, Robert,Klinga, Martti,Leskel?, Markku,Rieger, Bernhard
, p. 2321 - 2330 (2001)
Four new 1,4-diaza-2,3-dimethylbutadiene...
Spectroscopic and computational investigations of the thermodynamics of boronate ester and diazaborole self-assembly
Goldberg, Alexander R.,Northrop, Brian H.
, p. 969 - 980 (2016)
The solution phase self-assembly of boro...
Transition-Metal-Free Borylation of Aryl Bromide Using a Simple Diboron Source
Han, Min Su,Lim, Taeho,Ryoo, Jeong Yup
, p. 10966 - 10972 (2020/09/23)
In this study, we developed a simple tra...
Iron catalysis and water: A synergy for refunctionalization of boron
Wood, John L.,Marciasini, Ludovic D.,Vaultier, Michel,Pucheault, Mathieu
supporting information, p. 551 - 555 (2014/03/21)
A new catalytic system has been optimize...
Vaulted biaryls in catalysis: A structure-activity relationship guided tour of the immanent domain of the VANOL ligand
Guan, Yong,Ding, Zhensheng,Wulff, William D.
supporting information, p. 15565 - 15571 (2013/11/19)
The active site in the BOROX catalyst is...
123324-71-0 Process route
-
- 98-29-3
4-tert-Butylcatechol
-
- 123324-71-0
p-tert-butylphenylboronic acid
Conditions
| Conditions |
Yield |
|
In chloroform-d1; at 25 ℃; Equilibrium constant; Sealed tube;
|
|
-
-
potassium (4-tert-butyl)phenyltrifluoroborate
-
- 123324-71-0
p-tert-butylphenylboronic acid
Conditions
| Conditions |
Yield |
|
With 1H-imidazole; iron(III) chloride; at 20 ℃; for 0.25h; Inert atmosphere;
|
85% |
123324-71-0 Upstream products
123324-71-0 Downstream products
-
221018-01-5
4-(4-tert-butylphenyl)benzaldehyde
-
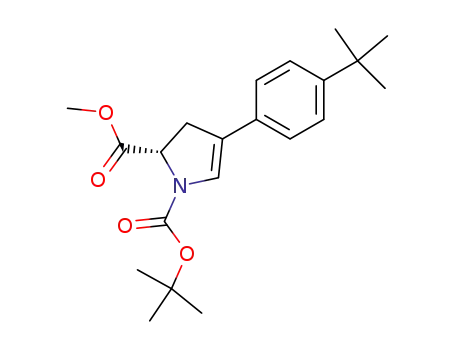
1027152-54-0
4-(4-tert-butyl-phenyl)-2,3-dihydro-pyrrole-1,2-dicarboxylic acid 1-tert-butyl ester 2-methyl ester
-
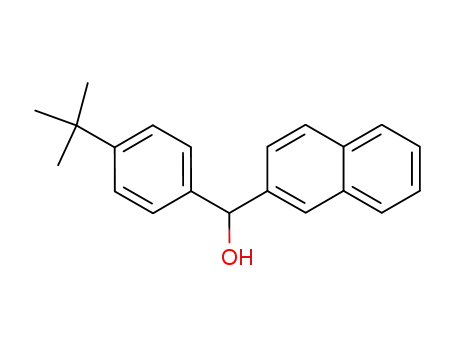
124419-75-6
(4-tert-Butylphenyl)(2-naphthyl)methanol
-
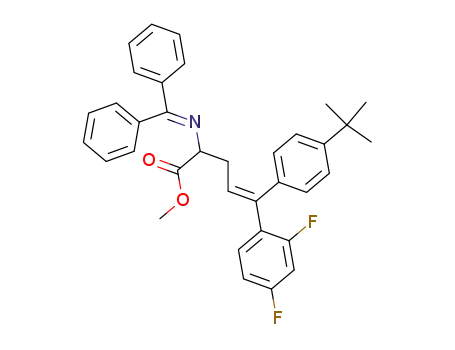
288101-17-7
2-(benzhydrylidene-amino)-5-(4-tert-butyl-phenyl)-5-(2,4-difluoro-phenyl)-pent-4-enoic acid methyl ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego