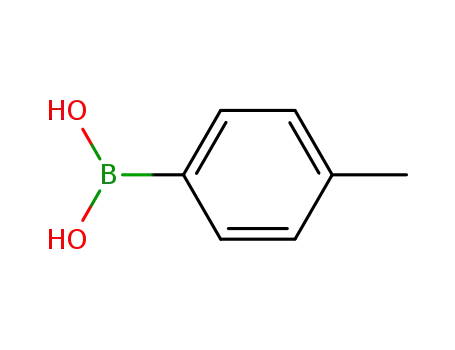
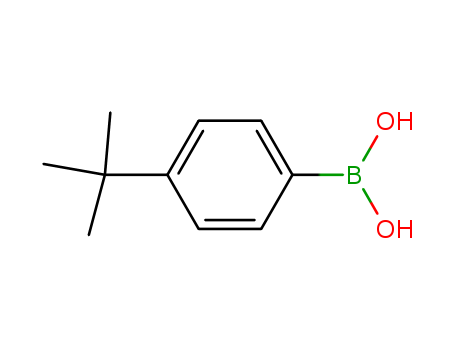
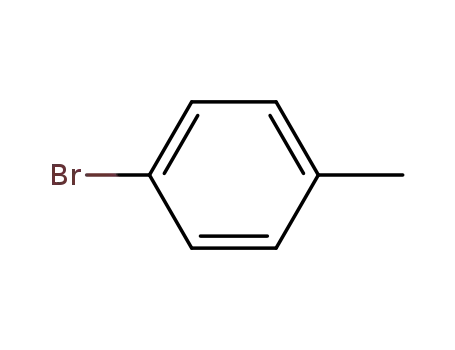Manufacturer supply high quality 4-Tolylboronic acid 5720-05-8 with ISO standards
- Molecular Formula:C7H9BO2
- Molecular Weight:135.958
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.976mmHg at 25°C
- Melting Point:256-263 °C(lit.)
- Refractive Index:1.505
- Boiling Point:275.2 °C at 760 mmHg
- PKA:8.84±0.10(Predicted)
- Flash Point:120.2 °C
- PSA:40.46000
- Density:1.1 g/cm3
- LogP:-0.32520
4-Tolylboronic acid(Cas 5720-05-8) Usage
InChI:InChI=1/C9H13N/c1-8(10-2)9-6-4-3-5-7-9/h3-8,10H,1-2H3/t8-/m1/s1
5720-05-8 Relevant articles
Atropisomerism of the C-1-C′-1 axis of 2,2′,8,8′-unsubstituted 1,1′-binaphthyl derivatives
Chow,Wan
, p. 5042 - 5047 (2001)
The Suzuki coupling of optically active ...
Bipyridinium and Phenanthrolinium Dications for Metal-Free Hydrodefluorination: Distinctive Carbon-Based Reactivity
Burton, Katherine I.,Elser, Iris,Waked, Alexander E.,Wagener, Tobias,Andrews, Ryan J.,Glorius, Frank,Stephan, Douglas W.
supporting information, p. 11730 - 11737 (2021/07/16)
The development of novel Lewis acids der...
Preparation method of trans-ketone intermediate
-
Paragraph 0086-0090; 0092, (2021/06/06)
The invention discloses a preparation me...
Aryl boronic acid preparation method
-
Paragraph 0029-0030, (2020/01/25)
The invention belongs to the technical f...
Palladium-catalyzed B-diarylation of diethylaminoborane for the synthesis of diarylborinic acids
Igarashi, Takuya,Shimazumi, Ryoma,Tobisu, Mamoru
supporting information, p. 760 - 763 (2020/07/10)
The palladium-catalyzed synthesis of dia...
5720-05-8 Process route
-
-
32203-14-8
2,3-dihydro-2-(4-methylphenyl)benzo[d][1,3,2]diazaborinin-4(1H)-one
-
-
5720-05-8
4-methylphenylboronic acid
-
-
28144-70-9,88-68-6
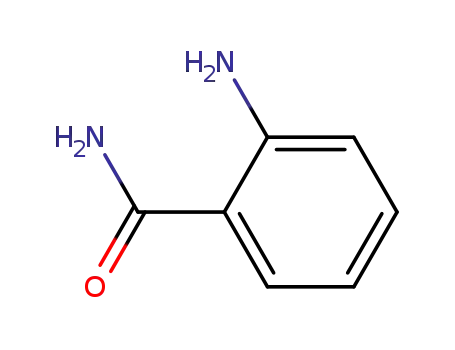
anthranilic acid amide
Conditions
| Conditions |
Yield |
|
In
1,4-dioxane; water;
at 140 ℃;
for 0.5h;
Microwave irradiation;
Sealed tube;
|
88%
78%
|
-
-
5419-55-6
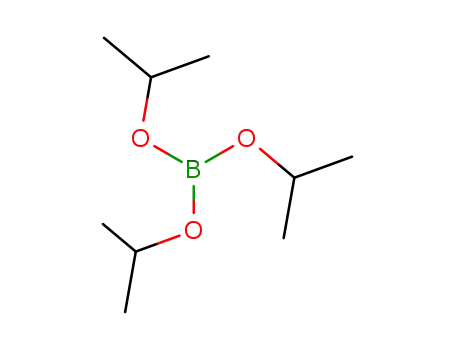
Triisopropyl borate
-
-
106-38-7
para-bromotoluene
-
-
5720-05-8
4-methylphenylboronic acid
Conditions
| Conditions |
Yield |
|
para-bromotoluene;
With
iodine; magnesium;
In
tetrahydrofuran;
at 70 ℃;
for 1h;
Triisopropyl borate;
In
tetrahydrofuran;
at 0 ℃;
for 1h;
Temperature;
Concentration;
Time;
|
91%
|
|
With
magnesium; ethylene dibromide;
In
tetrahydrofuran;
at 40 - 60 ℃;
for 6h;
Inert atmosphere;
|
58%
|
|
para-bromotoluene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 - -10 ℃;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at -78 - 20 ℃;
|
52%
|
|
With
hydrogenchloride; n-butyllithium;
In
tetrahydrofuran; hexane;
|
|
|
para-bromotoluene;
With
n-butyllithium;
In
tetrahydrofuran; hexane;
at -78 ℃;
for 0.25h;
Inert atmosphere;
Triisopropyl borate;
In
tetrahydrofuran; hexane;
at 25 ℃;
for 0.333333h;
Inert atmosphere;
|
|
5720-05-8 Upstream products
5720-05-8 Downstream products
-
34258-31-6
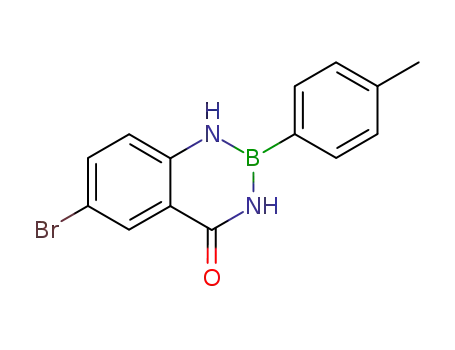
6-bromo-2-p-tolyl-2,3-dihydro-1H-benzo[1,3,2]diazaborinin-4-one
-
32203-14-8
2,3-dihydro-2-(4-methylphenyl)benzo[d][1,3,2]diazaborinin-4(1H)-one
-
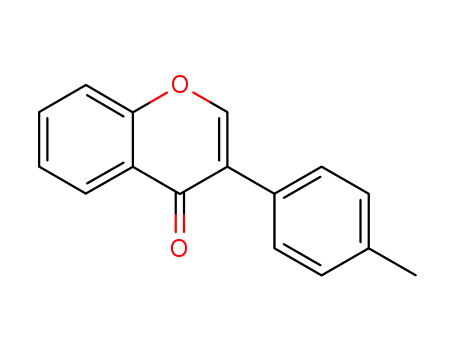
58113-12-5
3-(4-methylphenyl)-4H-chromen-4-one
-
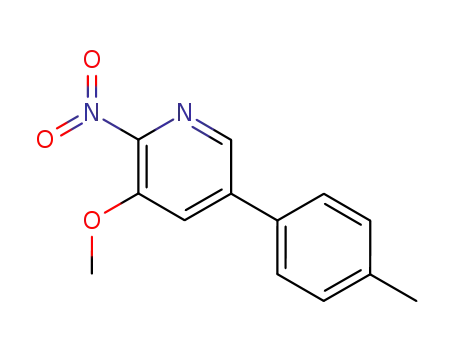
152684-18-9
3-methoxy-5-(4-methylphenyl)-2-nitropyridine
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego


![2,3-dihydro-2-(4-methylphenyl)benzo[d][1,3,2]diazaborinin-4(1H)-one](/upload/2025/4/df503acd-13c2-40a0-b81a-7f1ff47605cb.png)