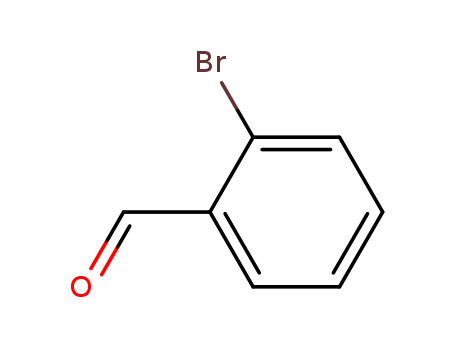
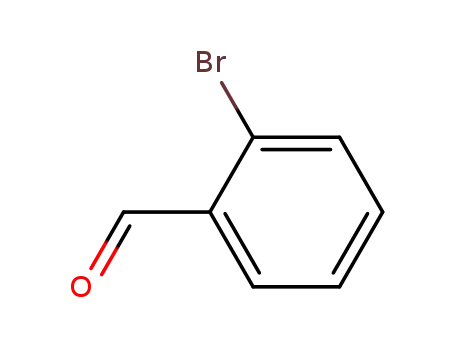
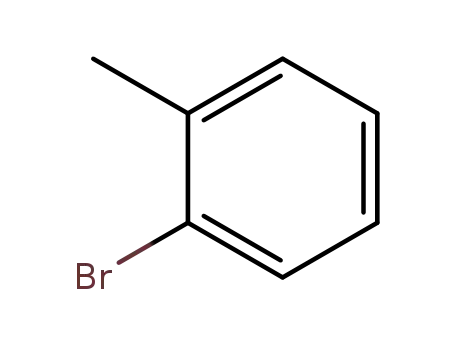99% Purity Commercial production 2-Bromobenzaldehyde 6630-33-7 with Cheapest Price
- Molecular Formula:C7H5BrO
- Molecular Weight:185.02
- Appearance/Colour:clear yellow liquid after melting
- Vapor Pressure:5.27E-11mmHg at 25°C
- Melting Point:21-22 °C
- Refractive Index:n20/D 1.595(lit.)
- Boiling Point:230.8 °C at 760 mmHg
- Flash Point:96.2 °C
- PSA:17.07000
- Density:1.58 g/cm3
- LogP:2.26160
2-Bromobenzaldehyde(Cas 6630-33-7) Usage
|
Chemical Description
|
2-bromobenzaldehyde is an organic compound with a molecular formula C7H5BrO. |
InChI:InChI=1/C17H15NO4S2/c1-20-12-5-6-14(21-2)11(8-12)9-15-16(19)18(17(23)24-15)10-13-4-3-7-22-13/h3-9H,10H2,1-2H3/b15-9+
6630-33-7 Relevant articles
Rhodium-Catalyzed Asymmetric Allenylation of Sulfonylimines and Application to the Stereospecific Allylic Allenylation
Sieber, Joshua D.,Angeles-Dunham, Veronica V.,Chennamadhavuni, Divya,Fandrick, Daniel R.,Haddad, Nizar,Grinberg, Nelu,Kurouski, Dimitry,Lee, Heewon,Song, Jinhua J.,Yee, Nathan K.,Mattson, Anita E.,Senanayake, Chris H.
, p. 3062 - 3068 (2016)
The rhodium-catalyzed asymmetric allenyl...
Nickel(II)-Catalyzed Selective (E)-Olefination of Methyl Heteroarenes Using Benzyl Alcohols via Acceptorless Dehydrogenative Coupling Reaction
Balamurugan, Gunasekaran,Ramesh, Rengan
, (2021/11/30)
An efficient catalytic protocol for the ...
METHOD FOR OXIDATIVE CLEAVAGE OF COMPOUNDS WITH UNSATURATED DOUBLE BOND
-
Paragraph 0071; 0077, (2021/07/10)
A method for oxidative cleavage of a com...
METHOD FOR OXIDATIVE CLEAVAGE OF COMPOUNDS WITH UNSATURATED DOUBLE BOND
-
Paragraph 0053-0054; 0062-0063, (2021/03/19)
A method for oxidative cleavage of a com...
New method for promoting photosensitive oxidation to remove 1, 2-mercaptoethanol acetal protecting group by utilizing visible light irradiation
-
Paragraph 0014-0016, (2021/01/30)
The invention discloses a new method for...
6630-33-7 Process route
-
-
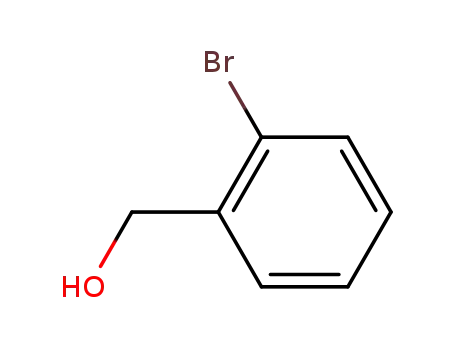
18982-54-2
o-bromobenzyl alcohol
-
-
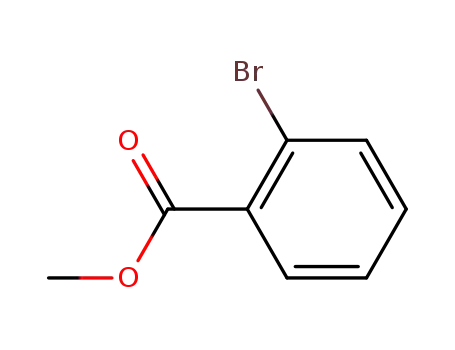
610-94-6
2-bromobenzoic acid methyl ester
-
-
6630-33-7
ortho-bromobenzaldehyde
Conditions
| Conditions |
Yield |
|
With
tert.-butylhydroperoxide; potassium iodide;
In
water;
at 65 ℃;
for 24h;
|
|
-
-
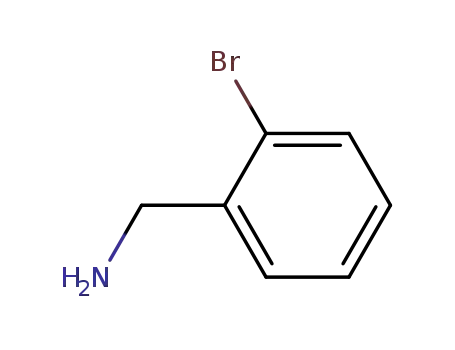
3959-05-5
2-bromobenzylamine
-
-
2042-37-7
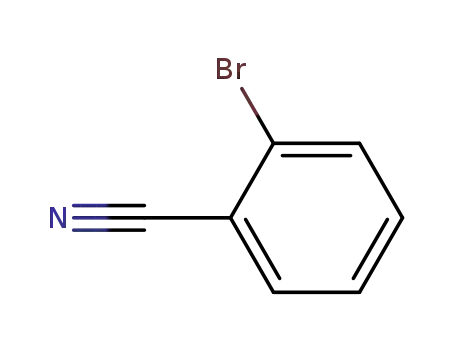
o-cyanobromobenzene
-
-
6630-33-7
ortho-bromobenzaldehyde
Conditions
| Conditions |
Yield |
|
With
iodine; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione;
In
dimethyl sulfoxide;
at 65 ℃;
for 1.5h;
|
68%
6 %Chromat.
|
6630-33-7 Upstream products
-
95-46-5
2-methylphenyl bromide
-
29418-67-5
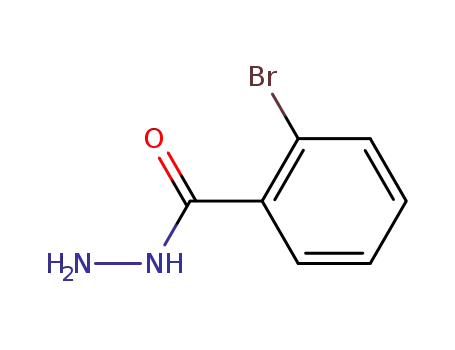
2-bromobenzohydrazide
-
2042-37-7
o-cyanobromobenzene
-
18982-54-2
o-bromobenzyl alcohol
6630-33-7 Downstream products
-
7345-79-1
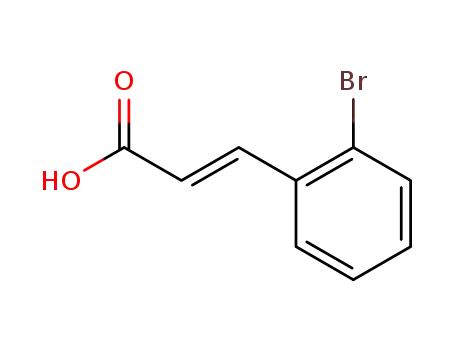
2-bromocinnamic acid
-
108062-08-4
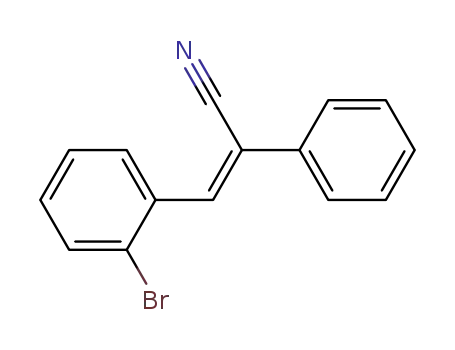
(Z)-3-(2-bromophenyl)-2-phenylacrylonitrile
-
55370-65-5
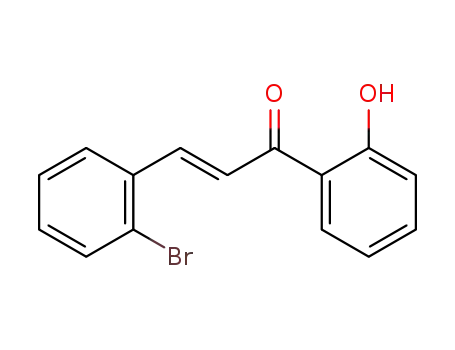
(E)-3-(2-bromophenyl)-1-(2'-hydroxyphenyl)prop-2-en-1-one
-
108791-79-3
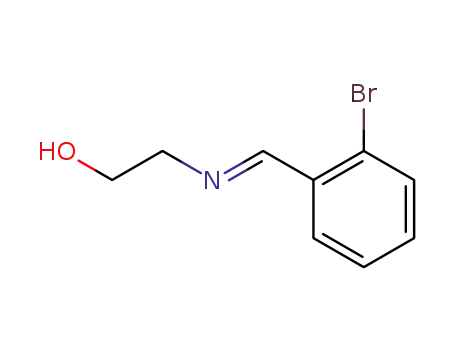
N-(2-bromobenzylidene)-2-hydroxyethylamine
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego