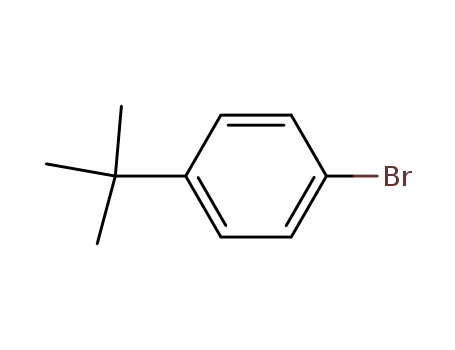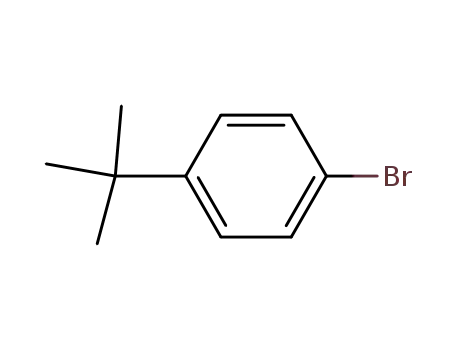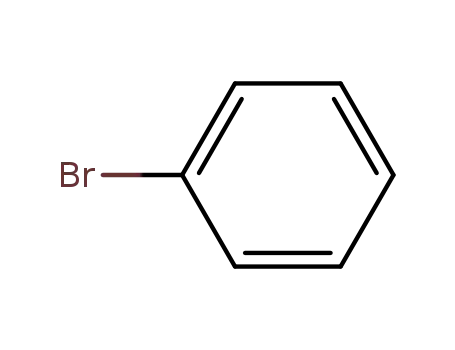Quality Factory Sells Top Purity 99% 1-Bromo-4-tert-butylbenzene 3972-65-4 with Safe Delivery
- Molecular Formula:C10H13Br
- Molecular Weight:213.117
- Appearance/Colour:clear colorless to slightly yellow liquid
- Vapor Pressure:0.0916mmHg at 25°C
- Melting Point:13-16 °C(lit.)
- Refractive Index:n20/D 1.533(lit.)
- Boiling Point:232 °C at 760 mmHg
- Flash Point:97.2 °C
- PSA:0.00000
- Density:1.236 g/cm3
- LogP:3.74660
3972-65-4 Relevant articles
Photochemical Sandmeyer-type Halogenation of Arenediazonium Salts
Belitz, Florian,Goo?en, Lukas J.,Manu Martínez, ángel,Schmid, Rochus,Sivendran, Nardana,Sowa Prendes, Daniel
supporting information, (2022/01/19)
Trihalide salts were found to efficientl...
Site-Specific Alkene Hydromethylation via Protonolysis of Titanacyclobutanes
Bartfield, Noah M.,Frederich, James H.,Law, James A.
supporting information, p. 14360 - 14364 (2021/05/27)
Methyl groups are ubiquitous in biologic...
Visible-Light-Photosensitized Aryl and Alkyl Decarboxylative Functionalization Reactions
Patra, Tuhin,Mukherjee, Satobhisha,Ma, Jiajia,Strieth-Kalthoff, Felix,Glorius, Frank
, p. 10514 - 10520 (2019/07/12)
Despite significant progress in aliphati...
Regioselective Halogenation of Arenes and Heterocycles in Hexafluoroisopropanol
Tang, Ren-Jin,Milcent, Thierry,Crousse, Benoit
, p. 930 - 938 (2018/01/28)
Regioselective halogenation of arenes an...
3972-65-4 Process route
-
-
176780-03-3,164927-39-3
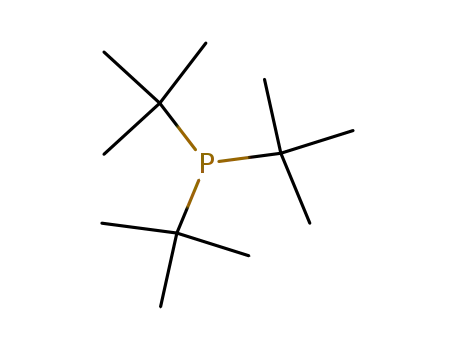
(P(o-tol)3)2Pd2(C6H4tBu-4)2Br2
-
-
13716-12-6
tri-tert-butyl phosphine
-
-
53199-31-8
bis(tri-t-butylphosphine)palladium(0)
-
-
3972-65-4
1-bromo-4-tert-butylbenzene
Conditions
| Conditions |
Yield |
|
In
benzene-d6;
byproducts: P(o-tol)3, biaryl, arene; in C6D6 soln. at 70°C; K(eq) = 3.3(6)E-4;
|
75%
|
-
-
253185-03-4,253185-04-5
tert-butylbenzene
-
-
3972-65-4
1-bromo-4-tert-butylbenzene
Conditions
| Conditions |
Yield |
|
With
bromine; iodine;
at 0 ℃;
|
100%
|
|
With
carbon dioxide; bromine;
at 40 ℃;
for 2h;
under 187519 Torr;
Supercritical conditions;
Green chemistry;
|
100%
|
|
With
benzyltrimethylazanium tribroman-2-uide; zinc(II) chloride;
In
acetic acid;
at 70 ℃;
for 2h;
|
95%
|
|
With
bromine fluoride;
In
ethanol; chloroform;
at -78 ℃;
for 0.0833333h;
|
89%
|
|
With
thallium (III) oxide; trifluoroacetic acid; potassium bromide;
at 20 ℃;
for 25h;
|
86%
|
|
With
Li2MnO3; bromine; oxygen;
at 80 ℃;
for 3h;
under 760.051 Torr;
Photolysis;
|
86%
|
|
With
bromine; iron;
In
tetrachloromethane;
at 0 ℃;
for 1h;
|
84%
|
|
With
bromine;
In
dichloromethane;
at 0 ℃;
Inert atmosphere;
|
80%
|
|
With
bromine; iron;
In
dichloromethane;
at 5 - 20 ℃;
for 24h;
|
71%
|
|
With
styrene-4-vinyl(N-alkylpyridinium bromide);
In
water; acetic acid;
at 85 ℃;
for 5h;
|
59%
|
|
With
bromine;
Sonnenlicht;
|
|
|
With
bromine; iron;
|
|
|
With
iodine;
durch Bromierung;
|
|
|
With
iron;
durch Bromierung;
|
|
|
With
bromine; iron;
|
|
|
With
bromine;
|
|
|
With
bromine; iodine; iron;
|
|
|
With
bromine; acetic anhydride; zinc(II) chloride;
In
acetic acid;
|
|
|
With
bromine; iron;
|
|
|
With
pyridine; bromine;
|
|
|
With
sodium metabisulfite; bromine;
Irradiation;
|
|
|
With
bromine fluoride; ethanol;
Yield given. Multistep reaction;
1.) CFCl3, -75 deg C; 2.) CHCl3, -75 deg C, 5-15 min;
|
|
|
With
bromine;
ZnBr2 on silica (100 Angstroem);
In
hexane;
at 25 ℃;
for 0.0333333h;
Yield given;
|
|
|
With
zeolite NaY; bromine;
In
dichloromethane;
at 20 ℃;
for 5h;
|
97 % Chromat.
|
|
Multi-step reaction with 2 steps
1: AlCl3 / 45 °C
2: iron-turnings; tetrachloromethane; bromine
With
tetrachloromethane; aluminium trichloride; bromine; iron;
|
|
|
With
N-Bromosuccinimide;
at 20 ℃;
for 16h;
regioselective reaction;
|
|
3972-65-4 Upstream products
-
108-86-1
bromobenzene
-
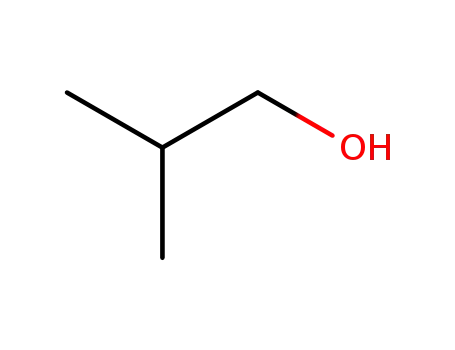
78-83-1
2-methyl-propan-1-ol
-
253185-03-4
tert-butylbenzene
-
1012-72-2
1,4-di-tert-butylbenzene
3972-65-4 Downstream products
-
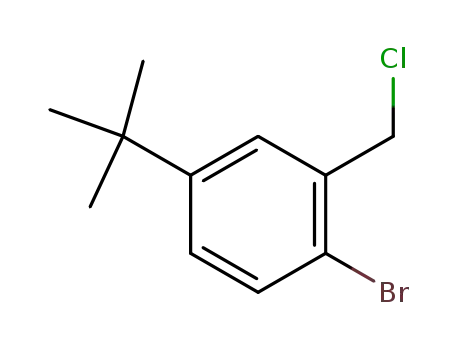
65276-29-1
4-Brom-3-chlormethyl-tert.-butyl-benzol
-
18412-68-5
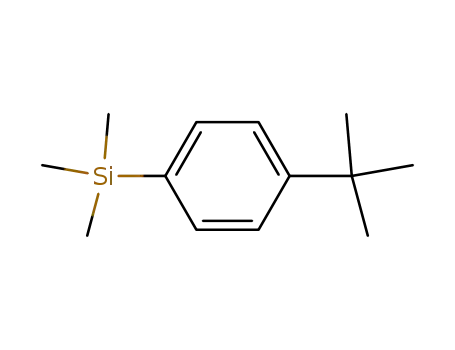
(4-tert-butylphenyl)trimethylsilane
-
66622-37-5
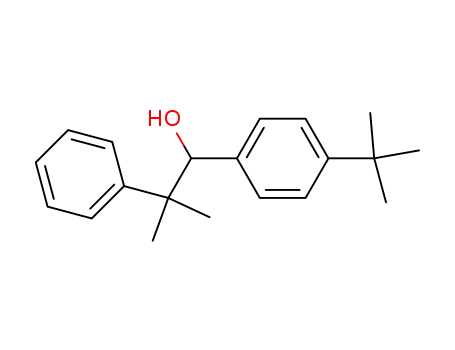
1-(4-tert-Butyl-phenyl)-2-methyl-2-phenyl-propan-1-ol
-
26110-91-8
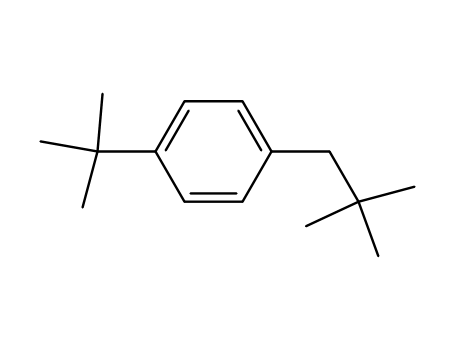
1-tert-Butyl-4-(2,2-dimethylpropyl)benzol
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego