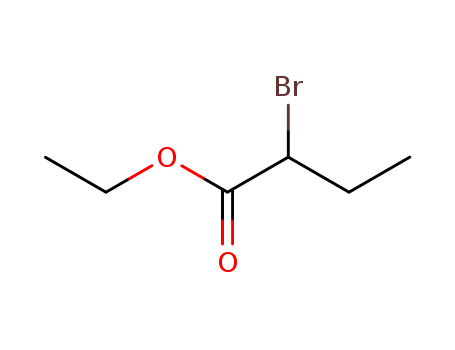
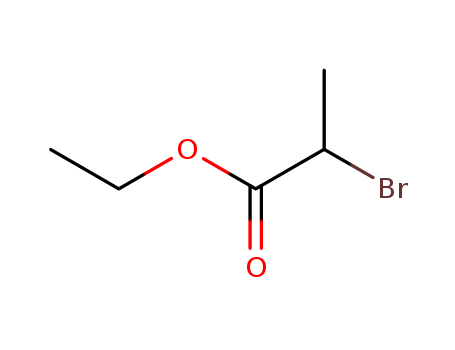
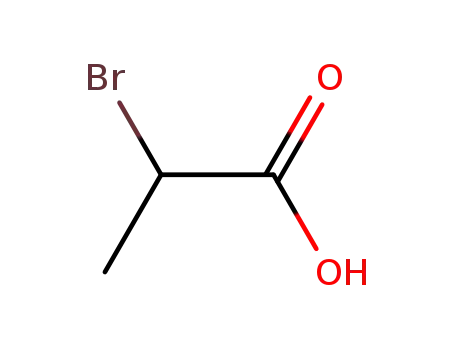
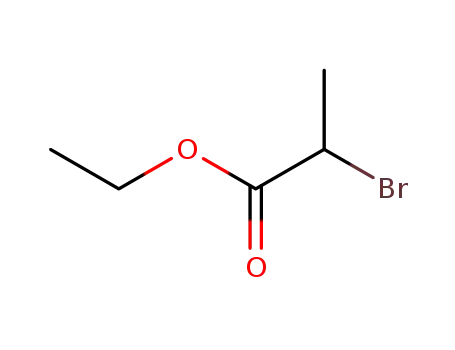
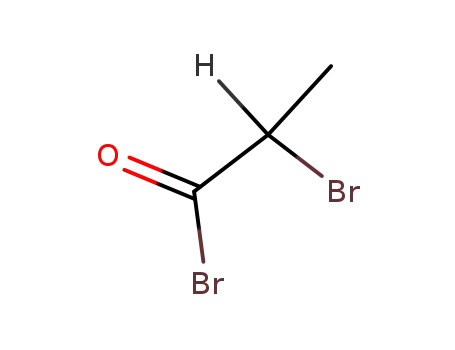
Excellent chemical plant bulk supply Ethyl (2S)-2-bromopropanoate 535-11-5
- Molecular Formula:C5H9BrO2
- Molecular Weight:181.029
- Appearance/Colour:clear colorless to very slightly yellow liquid
- Vapor Pressure:2.15mmHg at 25°C
- Melting Point:<25 °C
- Refractive Index:n20/D 1.446(lit.)
- Boiling Point:162.6 °C at 760 mmHg
- Flash Point:58.4 °C
- PSA:26.30000
- Density:1.413 g/cm3
- LogP:1.33290
Ethyl 2-bromopropionate(Cas 535-11-5) Usage
|
Chemical Description
|
Ethyl 2-bromopropionate is a chemical compound with the molecular formula C5H9BrO2. |
|
Purification Methods
|
Wash the ester with saturated aqueous Na2CO3 (three times), 50% aqueous CaCl2 (three times) and saturated aqueous NaCl (twice). Dry with MgSO4, CaCl2 or CaCO3, and distil it. [Beils |
InChI:InChI=1/C5H9BrO2/c1-3-8-5(7)4(2)6/h4H,3H2,1-2H3/t4-/m1/s1
535-11-5 Relevant articles
Acridine Orange Hemi(Zinc Chloride) Salt as a Lewis Acid-Photoredox Hybrid Catalyst for the Generation of α-Carbonyl Radicals
Das, Sanju,De Sarkar, Suman,Mandal, Tanumoy
supporting information, (2021/12/10)
A readily accessible organic-inorganic h...
Regio- and Stereoselective Nickel-Catalyzed Coupling of Boronic Acids with Allenoates
Liu, Yang,Daka, Mario,Bandini, Marco
, p. 3187 - 3196 (2018/08/17)
The Ni(II)-catalyzed cross-coupling of a...
Synthesis of [13C3]-B6 vitamers labelled at three consecutive positions starting from [13C3]-propionic acid
Bachmann, Thomas,Rychlik, Michael
, (2018/09/11)
[13C3]-labelled vitamers (PN, PL and PM)...
Prototypic 18F-Labeled Argininamide-Type Neuropeptide Y Y1R Antagonists as Tracers for PET Imaging of Mammary Carcinoma
Keller, Max,Maschauer, Simone,Brennauer, Albert,Tripal, Philipp,Koglin, Norman,Dittrich, Ralf,Bernhardt, Günther,Kuwert, Torsten,Wester, Hans-Jürgen,Buschauer, Armin,Prante, Olaf
supporting information, p. 304 - 309 (2017/03/17)
The neuropeptide Y (NPY) Y1 receptor (Y1...
535-11-5 Process route
-
-
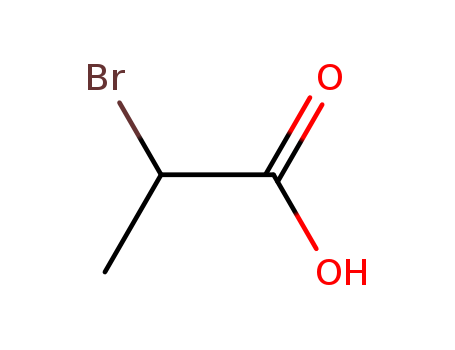
598-72-1,10327-08-9
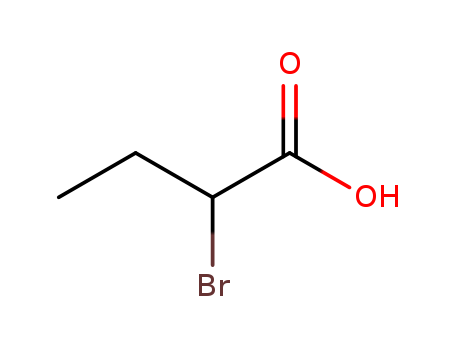
2-Bromopropionic acid
-
-
535-11-5,41978-69-2
Ethyl 2-bromopropionate
Conditions
| Conditions |
Yield |
|
With
Dowex 50 X8;
In
toluene;
for 5.5h;
Dean-Stark;
Reflux;
|
76%
|
|
With
hydrogenchloride;
In
diethyl ether;
|
75%
|
|
With
dmap; dicyclohexyl-carbodiimide;
In
diethyl ether;
at 25 ℃;
for 3h;
Inert atmosphere;
|
72%
|
|
With
hydrohalic acid;
|
|
|
With
sulfuric acid;
|
|
|
Acidic conditions;
|
|
-
-
563-76-8
α-bromopropionyl bromide
-
-
535-11-5,41978-69-2
Ethyl 2-bromopropionate
Conditions
| Conditions |
Yield |
|
|
|
|
|
|
|
With
pyridine;
In
chloroform;
at 0 ℃;
for 1h;
|
|
|
With
pyridine;
In
dichloromethane;
|
|
|
In
ethyl acetate;
at 20 ℃;
for 3h;
|
|
|
With
triethylamine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
|
With
pyridine;
In
dichloromethane;
at 0 - 20 ℃;
|
|
535-11-5 Upstream products
535-11-5 Downstream products
-
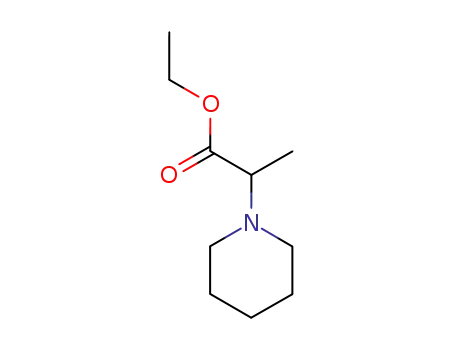
63909-12-6
2-piperidino-propionic acid ethyl ester
-
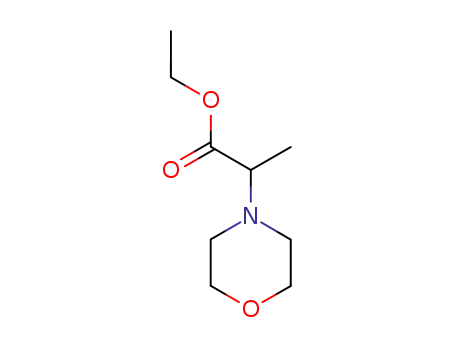
32418-62-5
(morpholinyl-4)-2 propanoate d'ethyle
-
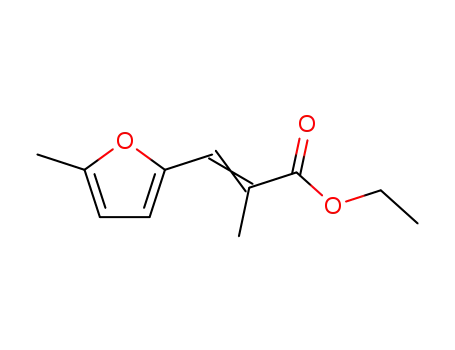
100058-86-4
ethyl 3-(5-methylfuran-2-yl)-2-methylacrylate
-
609-14-3
2-acetylpropanoic acid ethyl ester
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego