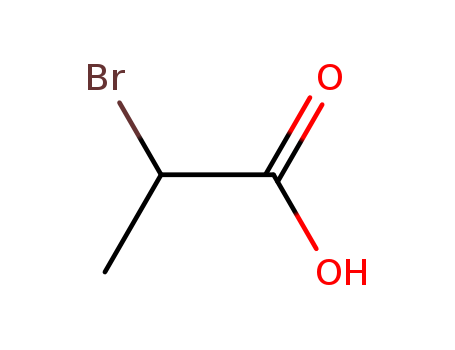
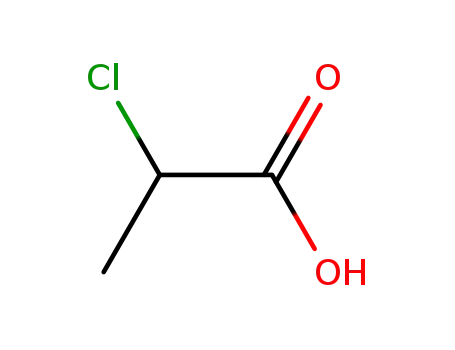
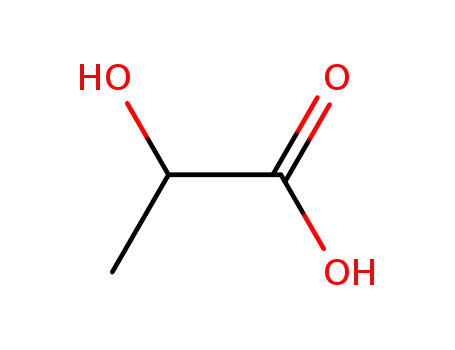
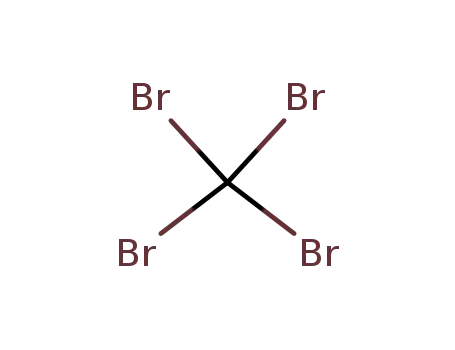
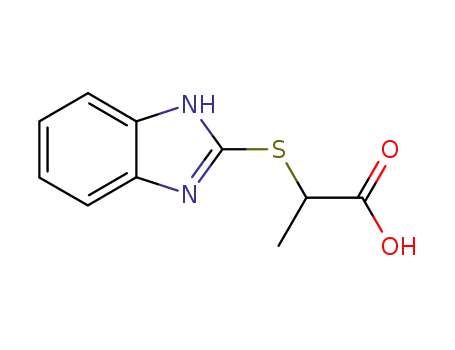
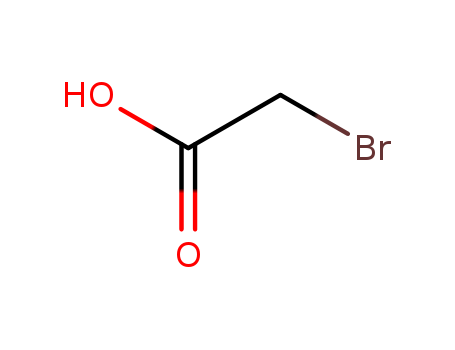
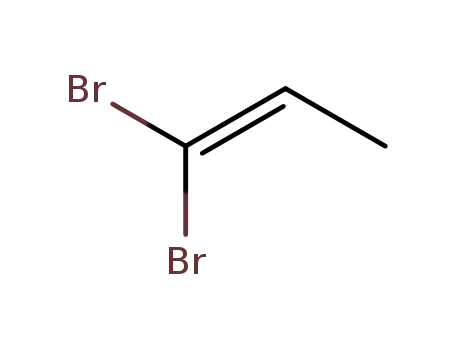Chemical plants supply high-quality 2-Bromopropionic Acid 598-72-1 in bulk
- Molecular Formula:C3H5BrO2
- Molecular Weight:152.975
- Appearance/Colour:clear colorless to yellowish liquid after melting
- Vapor Pressure:0.12mmHg at 25°C
- Melting Point:25.7 °C
- Refractive Index:n20/D 1.475(lit.)
- Boiling Point:202.6 °C at 760 mmHg
- PKA:pK1: 2.971 (25°C)
- Flash Point:76.3 °C
- PSA:37.30000
- Density:1.773 g/cm3
- LogP:0.85440
DL-2-Bromopropionic(Cas 598-72-1) Usage
|
Preparation
|
2-Bromopropionic acid is obtained by bromination of propionic acid. Add propionic acid and phosphorus trichloride to the reaction kettle, keep the temperature at 80°C, slowly add bromine to the reaction solution dropwise, and heat up to 85°C after the addition of bromine, and raise the temperature to 100°C when the color of bromine disappears completely, it takes about 2~3h, and then the bromine and hydrobromic acid are recovered under reduced pressure, and then the finished product is obtained by distillation under reduced pressure. |
|
Application
|
2-bromopropionic acid is an organic synthesis intermediate, used in the production of herbicides quizalofop-ethyl, napropamide, fenthiaprop and fungicides metalaxyl, benalaxyl, procymidone, etc., used in the synthesis of pharmaceutical intermediates body alanine. |
InChI:InChI=1/C3H5BrO2/c1-2(4)3(5)6/h2H,1H3,(H,5,6)/p-1/t2-/m0/s1
598-72-1 Relevant articles
1,3,2-Diazaphospholenes Catalyze the Conjugate Reduction of Substituted Acrylic Acids
Reed, John H.,Cramer, Nicolai
, p. 4262 - 4266 (2020/07/13)
The potent nucleophilicity and remarkabl...
Catalytic Bromination of Alkyl sp3C-H Bonds with KBr/Air under Visible Light
Zhao, Mengdi,Lu, Wenjun
supporting information, p. 5264 - 5267 (2018/09/12)
Alkyl sp3C-H bonds of cycloalkanes and f...
METHODS OF MAKING ACRYLIC ACID FROM LACTIC ACID OR ITS DERIVATIVES IN LIQUID PHASE
-
Page/Page column 66; 67, (2018/02/28)
Methods for making acrylic acid, acrylic...
METHOD OF MAKING ACRYLIC ACID FROM LACTIC ACID OR LACTIDE USING MOLTEN SALT CATALYSTS
-
Page/Page column 37; 38; 39; 41, (2018/09/28)
A method of making acrylic acid in liqui...
598-72-1 Process route
-
-
51-28-5
2,4-Dinitrophenol
-
-
598-72-1,10327-08-9
2-Bromopropionic acid
Conditions
| Conditions |
Yield |
|
With
water;
|
|
-
-
802294-64-0,79-09-4
propionic acid
-
-
590-92-1
3-Bromopropionic acid
-
-
107-94-8
chloropropionic acid
-
-
598-72-1,10327-08-9
2-Bromopropionic acid
-
-
598-78-7
(R,S)-2-chloropropionic acid
Conditions
| Conditions |
Yield |
|
With
hydrogenchloride; oxygen; potassium bromide; sodium nitrite;
In
chloroform; water;
at 40 ℃;
for 18h;
under 760.051 Torr;
Sealed tube;
Irradiation;
|
65%
23%
|
598-72-1 Upstream products
-
849585-22-4
LACTIC ACID
-
13195-80-7
1,1-dibromopropene
-
558-13-4
carbon tetrabromide
-
802294-64-0
propionic acid
598-72-1 Downstream products
-
5445-17-0
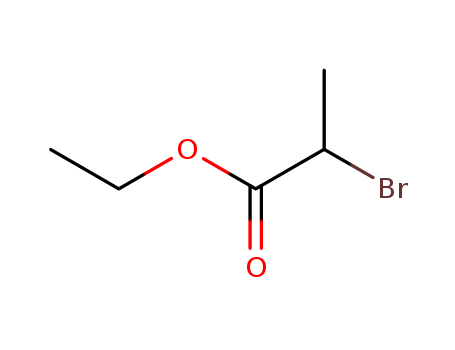
Methyl 2-bromopropionate
-
21547-70-6
2-(2-benzimidazolylthio)propionic acid
-
856810-90-7
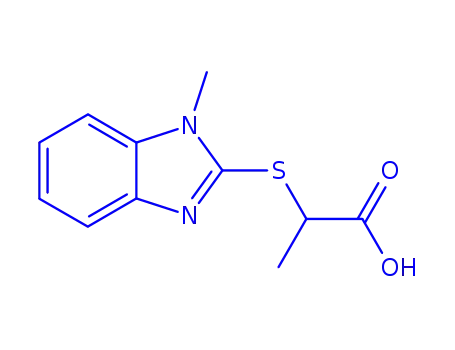
2-(1-methyl-1H-benzimidazol-2-ylmercapto)-propionic acid
-
6963-77-5
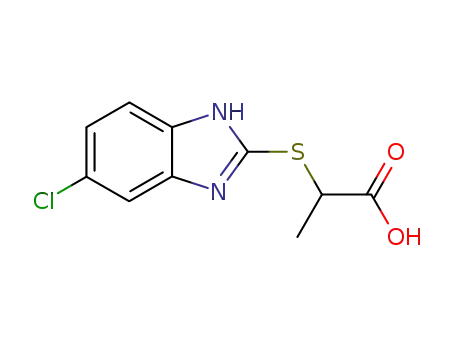
2-(5-chloro-1(3)H-benzimidazol-2-ylmercapto)-propionic acid
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego