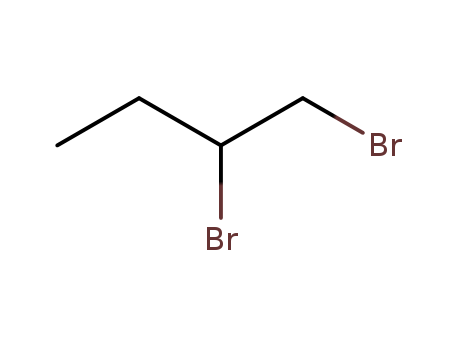
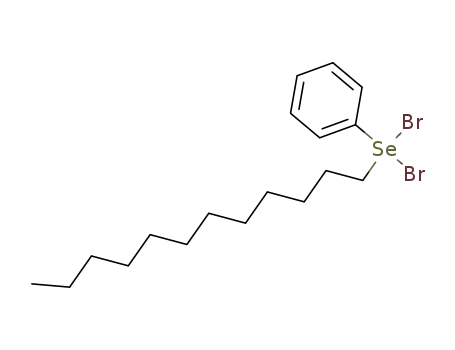Reputable manufacturer supply 1-Bromododecane 143-15-7 in stock with high standard
- Molecular Formula:C12H25Br
- Molecular Weight:249.234
- Appearance/Colour:Colorless transparent liquid
- Vapor Pressure:0.00817mmHg at 25°C
- Melting Point:-11 - -9 °C(lit.)
- Refractive Index:n20/D 1.458(lit.)
- Boiling Point:276.2 °C at 760 mmHg
- Flash Point:113 °C
- PSA:0.00000
- Density:1.039 g/cm3
- LogP:5.30220
1-Bromododecane(Cas 143-15-7) Usage
|
Preparation
|
1-Bromododecane is synthesized by the action of 1-dodecanol and hydrobromic acid. The 1-dodecanol is added to the enameled glass reaction pot, stirred and cooled, and sulfuric acid is slowly added. Stir for 1h, then add hydrobromic acid. The temperature is gradually increased to 90-95℃, and the reaction is stirred for 8h. After the reaction, the temperature is lowered to 30℃ and left for 8h. The acid layer is separated, and the oil layer is adjusted to pH 8 with dilute alkali solution, and the alkali solution is separated and washed with 50% ethanol. Distill under reduced pressure and collect 140-200℃ (3.33kPa) fractions to obtain refined 1-bromododecane. |
|
Application
|
1-Bromododecane is a halogenated hydrocarbon, mainly used in organic synthesis, and in medicine for the synthesis of disinfectant Benzyldodecyldimethylammonium Bromide, domiphen bromide, etc. |
|
General Description
|
1-Bromododecane is antimicrobial agent. |
InChI:InChI=1/C12H25Br/c1-2-3-4-5-6-7-8-9-10-11-12-13/h2-12H2,1H3
143-15-7 Relevant articles
Thiourea-Mediated Halogenation of Alcohols
Mohite, Amar R.,Phatake, Ravindra S.,Dubey, Pooja,Agbaria, Mohamed,Shames, Alexander I.,Lemcoff, N. Gabriel,Reany, Ofer
, p. 12901 - 12911 (2020/11/26)
The halogenation of alcohols under mild ...
Promotion of Appel-type reactions by N-heterocyclic carbenes
Hussein, Mohanad A.,Nguyen, Thanh Vinh
supporting information, p. 7962 - 7965 (2019/07/12)
N-Heterocyclic carbenes (NHCs) have been...
Nucleophilic Substitutions of Alcohols in High Levels of Catalytic Efficiency
Stach, Tanja,Dr?ger, Julia,Huy, Peter H.
supporting information, p. 2980 - 2983 (2018/05/28)
A practical method for the nucleophilic ...
METHOD OF CONVERTING ALCOHOL TO HALIDE
-
Page/Page column 182; 183; 185, (2017/01/02)
The present invention relates to a metho...
143-15-7 Process route
-
-
32294-60-3
diphenyl ditelluride
-
-
143-15-7
1-dodecylbromide
Conditions
| Conditions |
Yield |
|
With
sodium bromide;
In
N,N-dimethyl-formamide;
at 70 ℃;
Yield given;
|
|
-
-
143-15-7
1-dodecylbromide
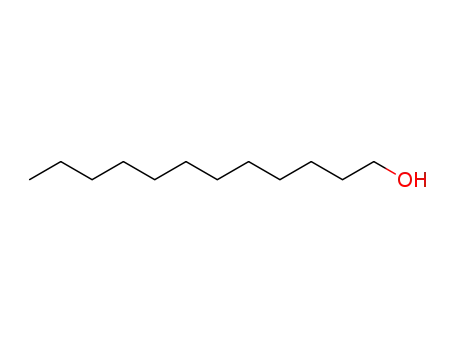
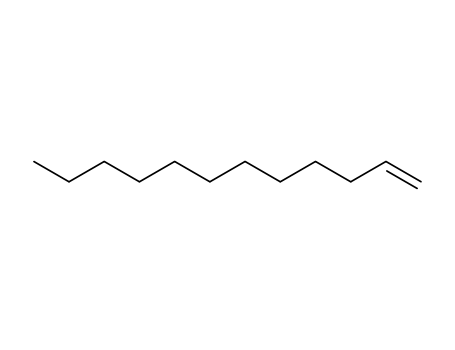
143-15-7 Upstream products
-
112-53-8
1-dodecyl alcohol
-
112-41-4
1-dodecene
-
102402-79-9
C18H30Br2Se
-
83486-03-7
C18H30Br2Te
143-15-7 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego