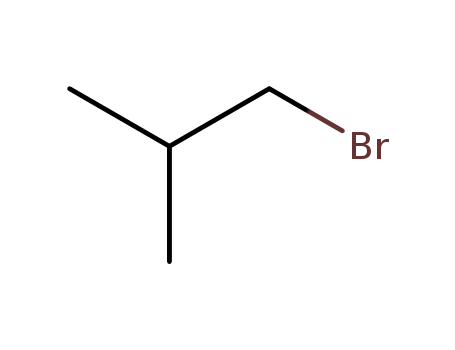
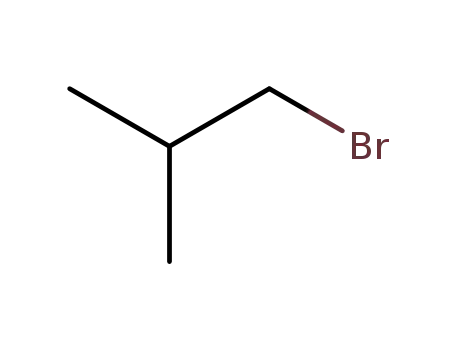
Reliable factory customized supply 1-Bromo-2-methylpropane 78-77-3
- Molecular Formula:C4H9Br
- Molecular Weight:137.019
- Appearance/Colour:clear liquid
- Vapor Pressure:41 hPa (20 °C)
- Melting Point:-119 °C
- Refractive Index:n20/D 1.435(lit.)
- Boiling Point:90.7 °C at 760 mmHg
- Flash Point:18.3 °C
- PSA:0.00000
- Density:1.26 g/cm3
- LogP:2.03730
1-Bromo-2-methylpropane(Cas 78-77-3) Usage
|
Safety Profile
|
Questionable
carcinogen with experimental neoplastigenic
data. Moderately toxic by intraperitoneal
route. A dangerous fire hazard when
exposed to heat or flame. When heated to
decomposition it emits toxic fumes of Br-.
See also BROMIDES. |
|
Purification Methods
|
Partially hydrolyse it to remove any tertiary alkyl halide, then fractionally distil it, then wash it with conc H2SO4, water and aqueous K2CO3, then redistil it from dry K2CO3. [Dunbar & Hammett J Am Chem Soc 72 109 1950, Beilstein 1 IV 294.] |
InChI:InChI=1/C4H9Br/c1-4(2)3-5/h4H,3H2,1-2H3
78-77-3 Relevant articles
-
Noller,Dinsmore
, p. 1025,1028, 1030 (1932)
-
New preparation method of febuxostat intermediate
-
Paragraph 0098-0100, (2020/03/06)
The invention relates to a new preparati...
PROCESSES FOR MAKING ALKYL HALIDES
-
Page/Page column 8, (2010/09/18)
The invention is directed to processes f...
Scavenger assisted combinatorial process for preparing libraries of tertiary amine compounds
-
, (2008/06/13)
This invention relates to a novel soluti...
Kinetics and thermochemistry of the R + HBr ? RH + Br (R = n-C3H7, isoC3H7, n-C4H9, isoC4H9, sec-C4H9 or tert-C4H9) equilibrium
Seetula, Jorma A.,Slagle, Irene R.
, p. 1709 - 1719 (2007/10/03)
The kinetics of the reactions of n-C3H7,...
78-77-3 Process route
-
-
75-28-5,40921-86-6
Isobutane
Conditions
| Conditions |
Yield |
|
With
norborn-2-ene; N-bromobis(trimethylsilyl)amine;
at 44.9 ℃;
for 2h;
Product distribution;
Mechanism;
Irradiation;
variation of temperature, time, reagent concentration, initiation method;
|
|
|
With
N-Bromosuccinimide;
In
dichloromethane;
at 15 ℃;
Product distribution;
Irradiation;
CH2CCl2; influence of additive - BrCCl3;
|
|
|
With
N-Bromosuccinimide; 1,1-Dichloroethylene;
In
dichloromethane;
at 14 - 15 ℃;
|
0.084 mmol
0.133 mmol
|
-
-
106-97-8,9003-29-6,9021-92-5
n-butane
-
-
78-76-2,5787-31-5
s-butyl bromide
Conditions
| Conditions |
Yield |
|
With
2AlBr3*CBr4; bromine;
at -20 ℃;
for 2h;
Title compound not separated from byproducts;
|
|
|
With
2AlBr3*CBr4; bromine;
In
various solvent(s);
at -20 ℃;
for 2h;
Yields of byproduct given. Title compound not separated from byproducts;
|
|
|
With
2AlBr3*CBr4; bromine;
at -20 ℃;
for 2h;
Yield given. Title compound not separated from byproducts;
|
|
|
With
2AlBr3*CBr4; bromine;
In
various solvent(s);
at -20 ℃;
for 2h;
Yield given. Title compound not separated from byproducts;
|
|
78-77-3 Upstream products
78-77-3 Downstream products
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego