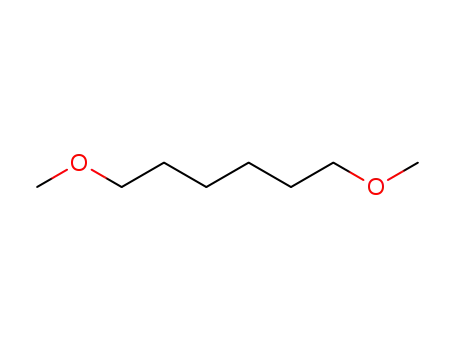
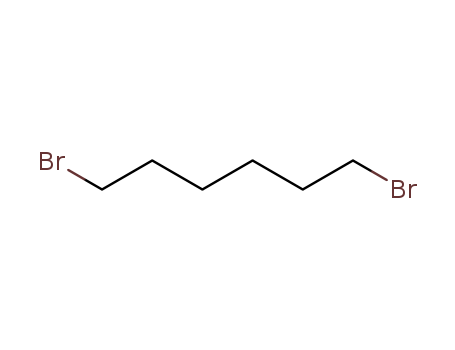
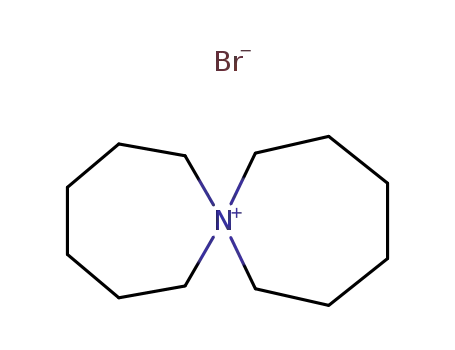Factory Export Top Purity 1,6-Dibromohexane 629-03-8 In Stock
- Molecular Formula:C6H12Br2
- Molecular Weight:243.969
- Appearance/Colour:Colorless or pale yellow liquid.
- Vapor Pressure:0.0483mmHg at 25°C
- Melting Point:-2-2.5 °C(lit.)
- Refractive Index:n20/D 1.507(lit.)
- Boiling Point:244.1 °C at 760 mmHg
- Flash Point:110.8 °C
- PSA:0.00000
- Density:1.584 g/cm3
- LogP:3.33660
1,6-Dibromohexane(Cas 629-03-8) Usage
|
Preparation
|
1,6-Dibromohexane is synthesized from hexanediol by bromination. |
InChI:InChI=1/C6H12Br2/c7-5-3-1-2-4-6-8/h1-6H2
629-03-8 Relevant articles
Stable and easily available sulfide surrogates allow a stereoselective activation of alcohols
Brutiu, Bogdan R.,Drescher, Martina,Matya?ovsky, Ján,Maulide, Nuno,Merad, Jérémy,Pinto, Alexandre,Stopka, Tobias
, p. 7770 - 7774 (2021/06/16)
Isothiouronium salts are easily accessib...
Triphenylphosphine-N-bromosuccinimide mediated chemoselective cyclodehydration of diols
Zhao, Shuo,Wu, You,Sun, Qi,Cheng, Tie-Ming,Li, Run-Tao
, p. 1154 - 1162 (2015/04/14)
A triphenylphosphine-N-bromosuccinimide ...
Biomolecule interaction using atomic force microscope
-
Page/Page column, (2014/03/26)
The present patent application describes...
Primary alkyl bromides from dimethylthiocarbamates
Moynihan, Meghan F.,Tucker, Joseph W.,Abelt, Christopher J.
experimental part, p. 3565 - 3568 (2009/06/18)
The conversion of primary alkyl dimethyl...
629-03-8 Process route
-
-
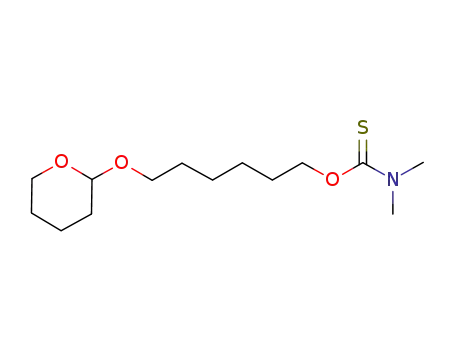
1110667-75-8
O-6-(tetrahydro-2H-pyran-2-yloxy)hexyl dimethylcarbamothioate
-
-
629-03-8
1 ,6-dibromohexane
-
-
53963-10-3
2-[(6-bromohexyl)oxy]tetrahydro-2H-pyran
-
-
4286-55-9
1-bromo-6-hexanol
Conditions
| Conditions |
Yield |
|
With
4-(bromomethylene)morpholin-4-ium bromide;
In
dichloromethane;
at 0 ℃;
for 1.5h;
Inert atmosphere;
|
27%
23%
14%
5%
|
-
-
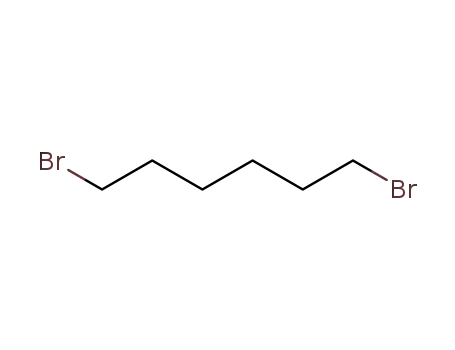
629-03-8
1 ,6-dibromohexane
-
-
4286-55-9
1-bromo-6-hexanol
Conditions
| Conditions |
Yield |
|
With
hydrogen bromide;
In
toluene;
for 13h;
Heating;
|
79%
|
|
With
hydrogen bromide;
In
toluene;
for 24h;
Heating;
|
69%
1%
|
629-03-8 Upstream products
-
629-11-8
1,6-hexanediol
-
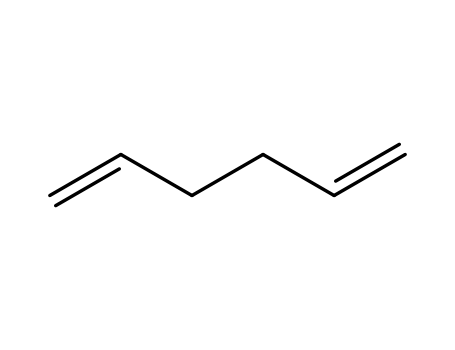
592-42-7
1,5-Hexadien
-
109-64-8
1,3-dibromo-propane
-
10035-10-6
hydrogen bromide
629-03-8 Downstream products
-
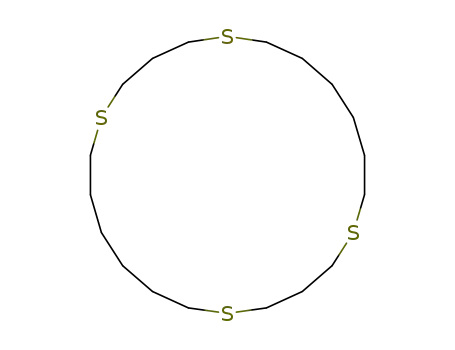
52559-88-3
1,5,12,16-tetrathia-cyclodocosane
-
56496-17-4
7-azoniaspiro<6.6>tridecane bromide
-
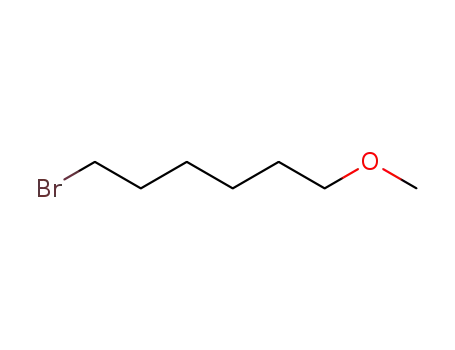
50592-87-5
1-bromo-6-methoxyhexane
-
13179-98-1
1,6-dimethoxyhexane
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego

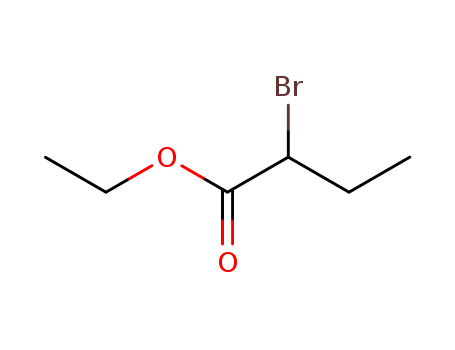
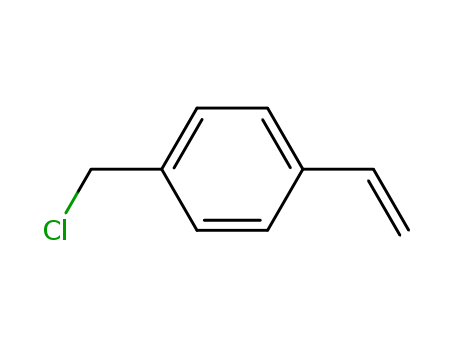



![2-[(6-bromohexyl)oxy]tetrahydro-2H-pyran](/upload/2025/4/2cd5280e-8fa6-49d7-a731-bb015850aeaa.png)