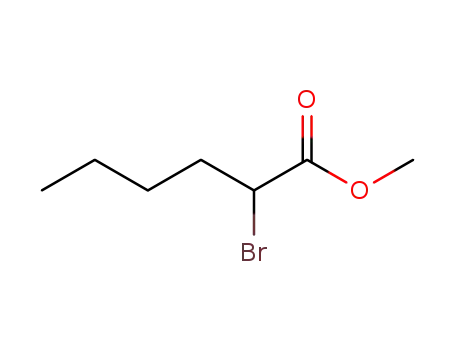
Top Quality Chinese Manufacturer supply 5445-19-2 Methyl 2-bromohexanoate
- Molecular Formula:C7H13BrO2
- Molecular Weight:209.083
- Appearance/Colour:Clear, colourless liquid
- Vapor Pressure:0.411mmHg at 25°C
- Melting Point:< -10ºC
- Refractive Index:n20/D 1.455
- Boiling Point:195.9 °C at 760 mmHg
- Flash Point:80.6 °C
- PSA:26.30000
- Density:1.297 g/cm3
- LogP:2.11310
Methyl 2-bromohexanoate(Cas 5445-19-2) Usage
|
General Description
|
Methyl 2-bromohexanoate undergoes addition reaction with methyl-10-undecenoate to yield lactone and dimethyl-2-butyltridecandioate. |
InChI:InChI=1/C7H13BrO2/c1-3-4-5-6(8)7(9)10-2/h6H,3-5H2,1-2H3
5445-19-2 Relevant articles
Diastereo- And Enantioselective Synthesis of Quaternary α-Amino Acid Precursors by Copper-Catalyzed Propargylation
Zhu, Qiongqiong,Meng, Beibei,Gu, Congzheng,Xu, Ye,Chen, Jie,Lei, Chuanhu,Wu, Xiaoyu
supporting information, p. 9985 - 9989 (2019/12/24)
A diastereo- and enantioselective propar...
One-pot oxidative bromination – Esterification of aldehydes to 2-bromoesters using cerium (IV) ammonium nitrate and lithium bromide
Nikishin, Gennady I.,Kapustina, Nadezhda I.,Sokova, Lyubov L.,Bityukov, Oleg V.,Terent'ev, Alexander O.
supporting information, p. 352 - 354 (2017/01/03)
A two-step, one-pot reaction of aldehyde...
Site-selective aliphatic C-H bromination using N -bromoamides and visible light
Schmidt, Valerie A.,Quinn, Ryan K.,Brusoe, Andrew T.,Alexanian, Erik J.
supporting information, p. 14389 - 14392 (2014/12/10)
Transformations that selectively functio...
GC separation of enantiomers of alkyl esters of 2-bromo substituted carboxylic acids enantiomers on 6-tbdms-2,3-di-alkyl- β- And γ-cyclodextrin stationary phases
Spanik, Ivan,Kaceriakova, Darina,Krupcik, Jan,Armstrong, Daniel Wayne
, p. 279 - 285 (2014/06/09)
The gas chromatographic separation of en...
5445-19-2 Process route
-
-
5445-19-2
methyl 2-bromohexanoate
-
-
106-70-7
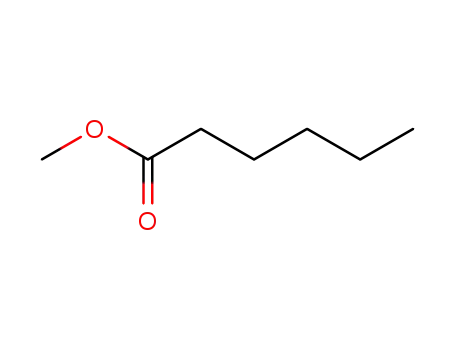
methyl hexanoate
Conditions
| Conditions |
Yield |
|
hexanal;
With
ammonium cerium (IV) nitrate; lithium bromide;
In
neat liquid;
methanol;
In
neat liquid;
at 35 - 40 ℃;
for 3.5h;
|
80%
10 %Chromat.
|
-
-
42768-46-7
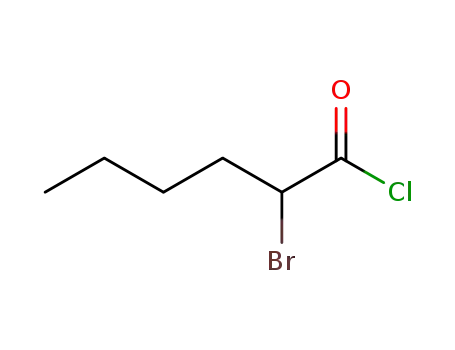
2-bromohexanoyl chloride
-
-
5445-19-2
methyl 2-bromohexanoate
Conditions
| Conditions |
Yield |
|
With
triethylamine; mercury;
In
methanol; benzene;
|
69.7%
|
5445-19-2 Upstream products
-
186581-53-3
diazomethane
-
616-05-7
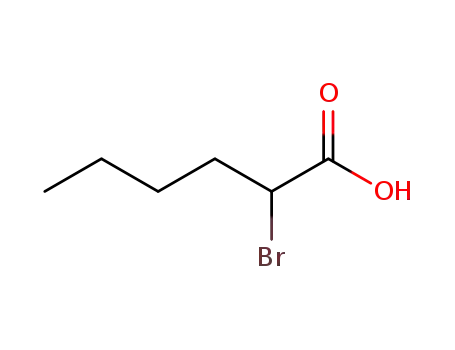
2-bromohexanoic acid
-
67-56-1
methanol
-
42768-46-7
2-bromohexanoyl chloride
5445-19-2 Downstream products
-
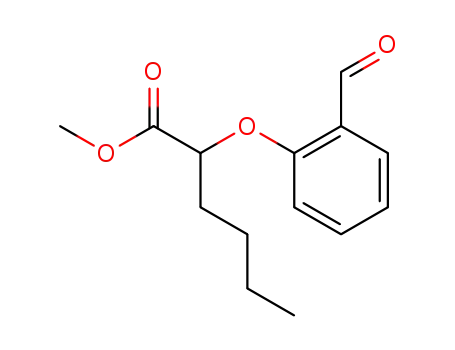
138320-26-0
methyl 2-(2-formylphenoxy)hexanoate
-
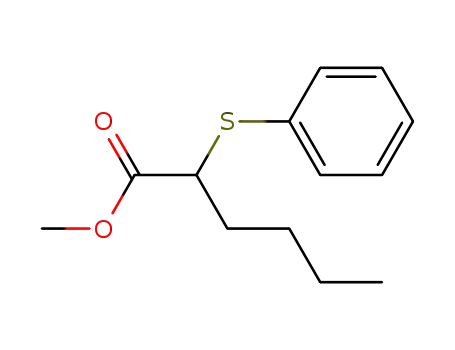
75280-16-9
methyl 2-(phenylthio)hexanoate
-
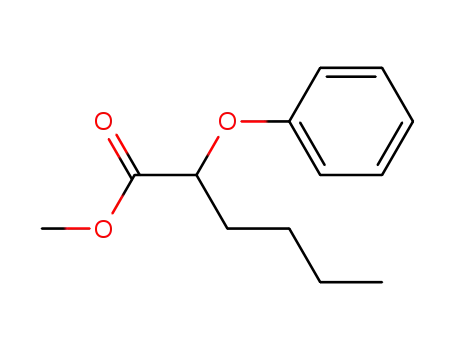
27610-91-9
2-Phenoxy-hexanoic acid methyl ester
-
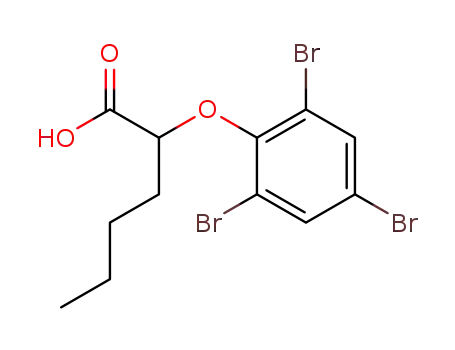
4592-76-1
2-(2,4,6-Tribrom-phenoxy)-capronsaeure
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego