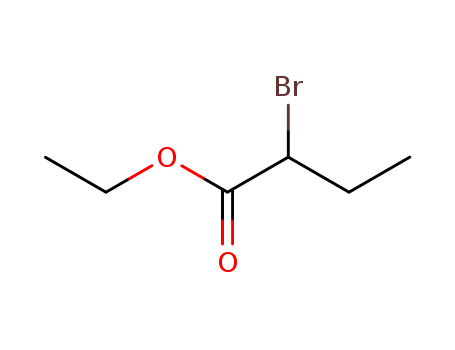
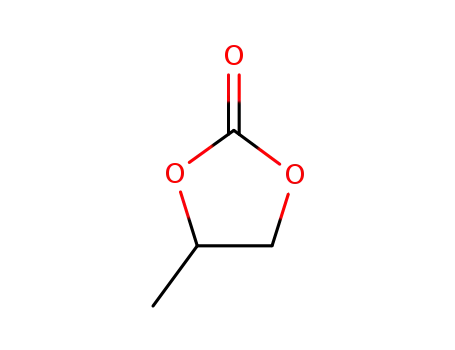
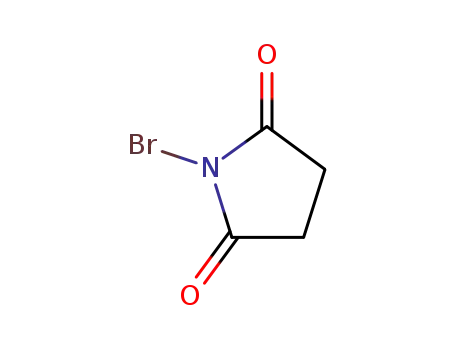
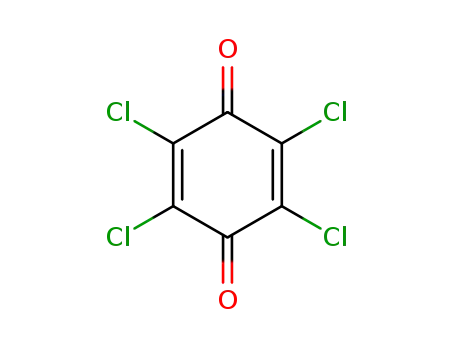
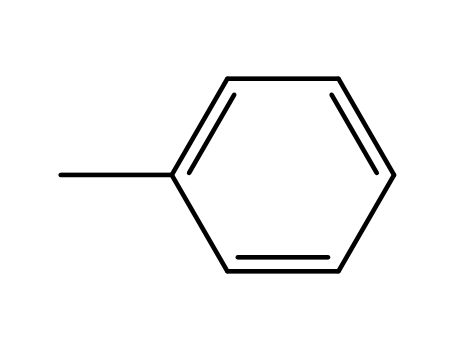
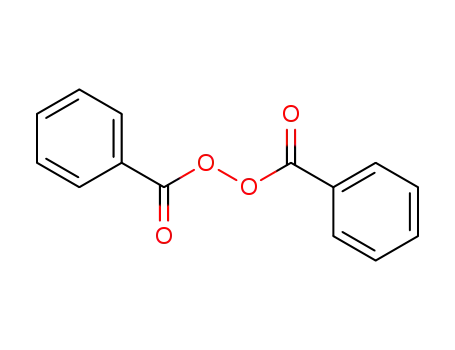
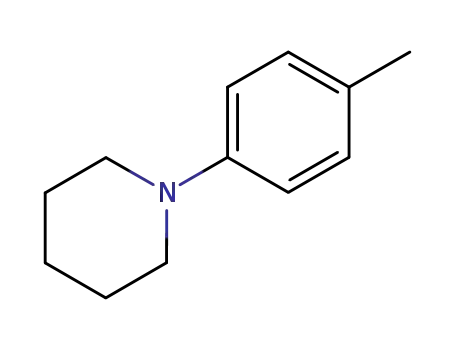
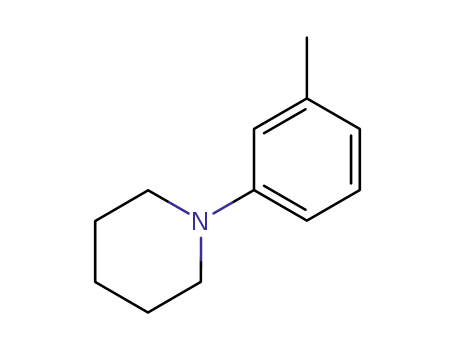
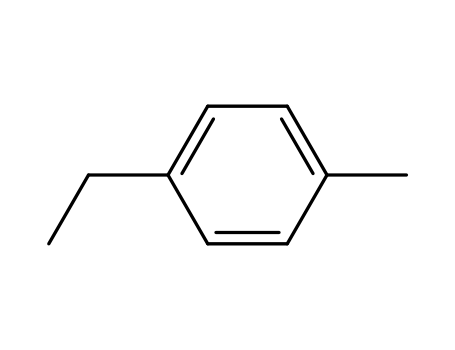
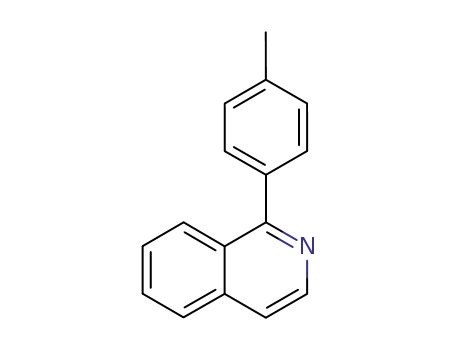
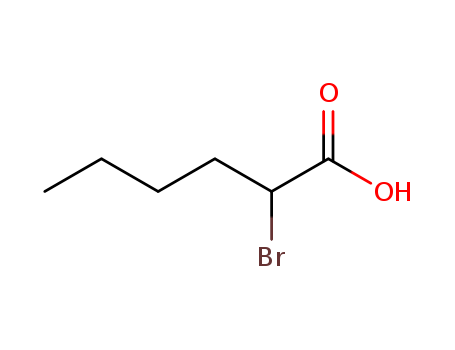
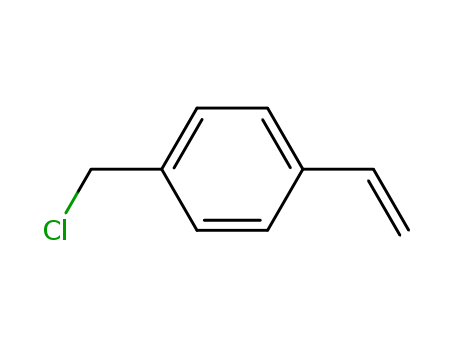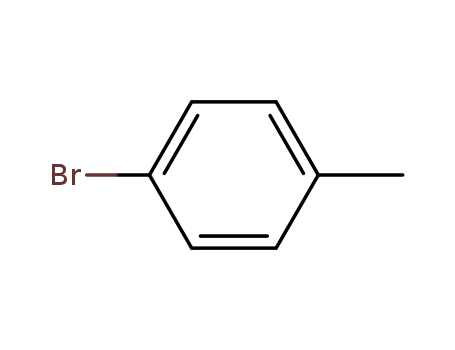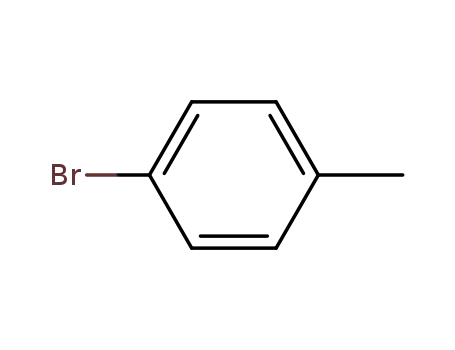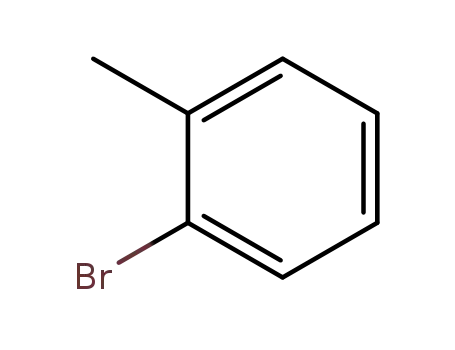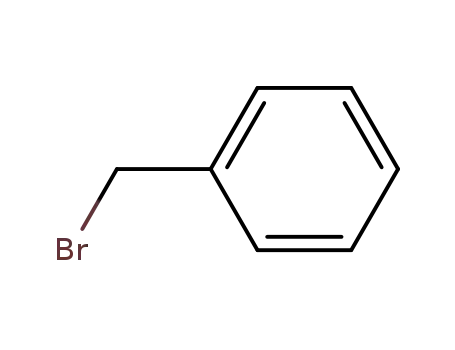Novel site-specific one-step bromination of substituted benzenes
Srivastava, Sanjay K.,Chauhan, Prem Man Singh,Bhaduri, Amiya P.
, p. 2679 - 2680 (1996)
Regiospecific bromination of benzene der...
A simple and improved procedure for selective ring bromination of alkyl-substituted aromatic hydrocarbons on the surface of alumina
Ranu,Sarkar,Chakraborty
, p. 1095 - 1099 (1992)
Highly selective ring bromination of alk...
Selective Halogenation of Aromatic Hydrocarbons with Alumina-Supported Copper(II) Halides
Kodomari, Mitsuo,Satoh, Hiroaki,Yoshitomi, Suehiko
, p. 2093 - 2094 (1988)
-
Induced bromination of aromatic hydrocarbons by alkali metals bromides and sodium hypochlorite
Sadygov,Alimardanov,Chalabiev
, p. 1631 - 1636 (2005)
Induced bromination of aromatic compound...
-
Brown,McGary
, p. 2310 (1955)
-
-
de la Mare,Harvey
, p. 36,38 (1956)
-
Bromination of alkylbenzenes in 1-butyl-3-methylimidazolium bromide and its dibromide complex
Gruzdev,Virzum,Krylov
, p. 263 - 267 (2010)
Alkylbenzenes were subjected to brominat...
Deoxygenation of carbonyl compounds using an alcohol as an efficient reducing agent catalyzed by oxo-rhenium complexes
Bernardo, Joana R.,Fernandes, Ana C.
, p. 2675 - 2681 (2016)
This work describes the first methodolog...
Regioselective bromination of aromatic compounds with Br 2/SO2Cl2 over microporous catalysts
Gnaim, Jallal M.,Sheldon, Roger A.
, p. 4465 - 4468 (2005)
A new selective brominating system Br2/S...
A new method of bromination of aromatic rings by an iso-amyl nitrite/HBr system
Gavara, Laurent,Boisse, Thomas,Rigo, Beno?t,Hénichart, Jean-Pierre
, p. 4999 - 5004 (2008)
A mixture of iso-amyl nitrite/HBr is sho...
Reactivities of Several Nucleophiles toward 4-Methylbenzyne in Very Hot Water
Zoratti, Mario,Bunnett, J. F
, p. 1776 - 1782 (1980)
By means of competition experiments, the...
-
Henne,Zimmer
, p. 1362 (1951)
-
Halogenation of Aromatic Compounds by using Sodium Perborate as an Oxidant
Deshmukh, Anjali P.,Padiya, Kamlesh J.,Jadhav, Vidyadhar K.,Salunkhe, Manikrao M.
, p. 828 - 829 (1998)
A facile method for the halogenation of ...
Bipyridinium and Phenanthrolinium Dications for Metal-Free Hydrodefluorination: Distinctive Carbon-Based Reactivity
Burton, Katherine I.,Elser, Iris,Waked, Alexander E.,Wagener, Tobias,Andrews, Ryan J.,Glorius, Frank,Stephan, Douglas W.
, p. 11730 - 11737 (2021)
The development of novel Lewis acids der...
Dynamics of endoergic substitution reactions. I. Br+chlorinated aromatic compounds
Robinson, Gary N.,Continetti, Robert E.,Lee, Yuan T.
, p. 6226 - 6237 (1988)
The endoergic substitution reactions Br ...
-
Brown,Stock
, p. 1421,1422 (1957)
-
Selective para-Bromination of Toluene catalysed by Na-Y Zeolite in the Presence of an Epoxide
Vega, Fernando de la,Sasson, Yoel
, p. 653 - 654 (1989)
Propylene oxide, serving as an HBr scave...
Halogenation Using N-Halogenocompounds. II. Acid Catalyzed Bromination of Aromatic Compounds with 1,3-Dibromo-5,5-dimethylhydantoin
Eguchi, Hisao,Kawaguchi, Hideichiro,Yoshinaga, Sachiyo,Nishida, Akiko,Nishiguchi, Takeshi,Fujisaki, Shizuo
, p. 1918 - 1921 (1994)
Ring bromination of aromatic compounds u...
Induced bromination of aromatic hydrocarbons with alkali metal bromides in the presence of oxidants
Sadygov,Alimardanov,Chalabiev
, p. 949 - 956 (2006)
The features of induced bromination of a...
Convenient Synthesis of Aryl Halides from Arylamines via Treatment of 1-Aryl-3,3-dialkyltriazenes with Trimethylsilyl Halides
Ku, Hao,Barrio, Jorge R.
, p. 5239 - 5241 (1981)
-
Synthesis of tin-containing cyclodextrins as potential enzyme models
Zhou, You,Marinescu, Lavinia,Pedersen, Christian Marcus,Bols, Mikael
, p. 6383 - 6389,7 (2012)
The synthesis and the characterization o...
Room-temperature catalytic hydrodefluorination of C(sp3)-F bonds
Scott, Valerie J.,Celenligil-Cetin, Remle,Ozerov, Oleg V.
, p. 2852 - 2853 (2005)
Room-temperature catalytic hydrodefluori...
Molecular structures of the metastable charge-transfer complexes of benzene (and toluene) with bromine as the pre-reactive intermediates in electrophilic aromatic bromination
Vasilyev, Alexandr V.,Lindeman, Sergey V.,Kochi, Jay K.
, p. 582 - 592 (2002)
Successful crystallization and X-ray cry...
-
Bunce
, p. 664,668 (1972)
-
1,3-dibromo-5,5-dimethylhydantoin, a useful reagent for aromatic bromination
Chassaing, Christophe,Haudrechy, Arnaud,Langlois, Yves
, p. 4415 - 4416 (1997)
1,3-dibromo-5,5-dimethylhydantoin (DBDMH...
OOxidative bromination of activated aromatic compounds using aqueous nitric acid as an oxidant
Joshi, Ashutosh V.,Baidossi, Mubeen,Mukhopadhyay, Sudip,Sasson, Yoel
, p. 568 - 570 (2004)
Oxidative bromination of activated aroma...
An efficient copper-catalysed aerobic oxybromination of arenes in water
Wang, Jian,Wang, Wei,Li, Jing-Hua
, p. 2124 - 2126 (2010)
An aerobic oxybromination of arenes cata...
Direct bromodeboronation of arylboronic acids with CuBr2 in water
Tang, Yan-Ling,Xia, Xian-Song,Gao, Jin-Chun,Li, Min-Xin,Mao, Ze-Wei
supporting information, (2021/01/05)
An efficient and practical method has be...
Poly-N-bromosulfonamide-melamine as a novel brominating reagent for regioselective ipso-bromination of arylboronic acids
Alavinia, Sedigheh,Ghorbani-Vaghei, Ramin
, p. 1269 - 1276 (2021/08/27)
A practical synthetic method for the syn...
Hydrogen-Bond-Donor Solvents Enable Catalyst-Free (Radio)-Halogenation and Deuteration of Organoborons
Yang, Yi,Gao, Xinyan,Zeng, Xiaojun,Han, Junbin,Xu, Bo
supporting information, p. 1297 - 1300 (2020/12/23)
A hydrogen bond donor solvent assisted (...
The graphite-catalyzed: ipso -functionalization of arylboronic acids in an aqueous medium: metal-free access to phenols, anilines, nitroarenes, and haloarenes
Badgoti, Ranveer Singh,Dandia, Anshu,Parewa, Vijay,Rathore, Kuldeep S.,Saini, Pratibha,Sharma, Ruchi
, p. 18040 - 18049 (2021/05/29)
An efficient, metal-free, and sustainabl...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego