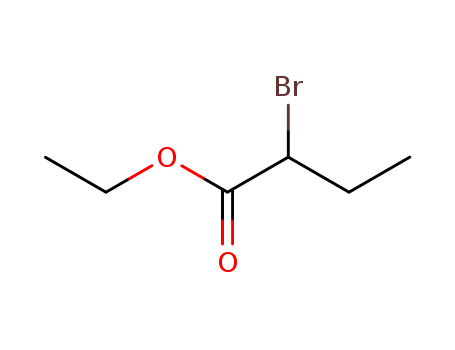
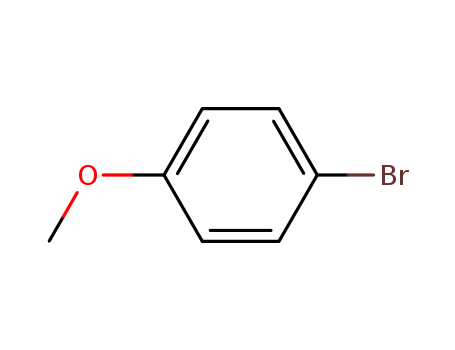
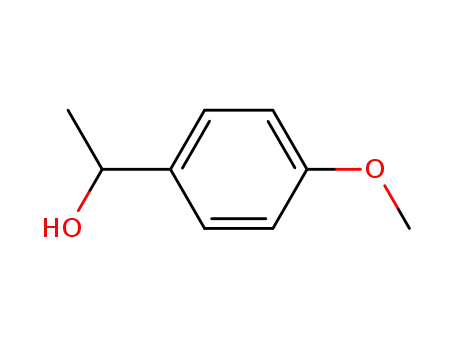
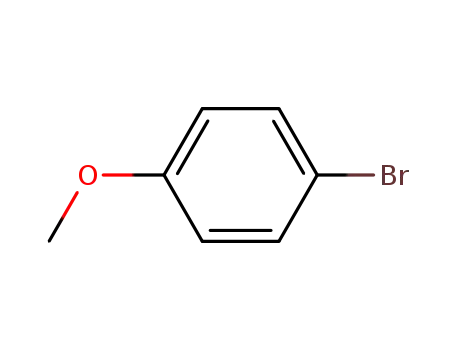
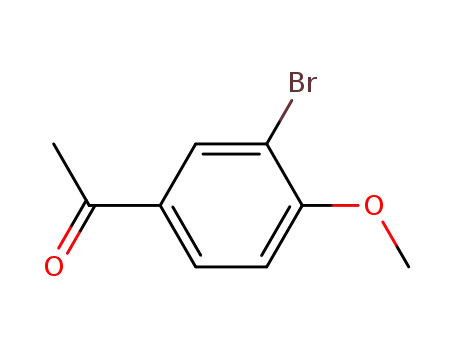
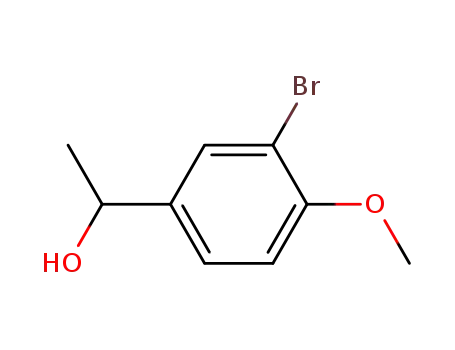
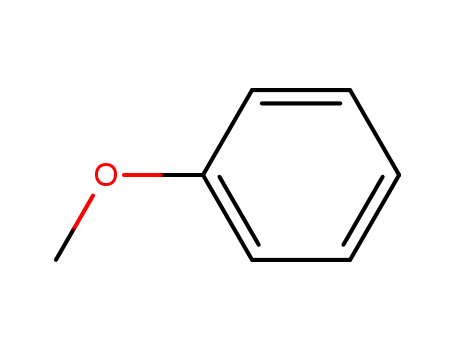
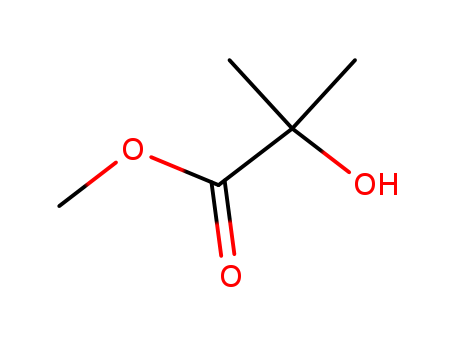
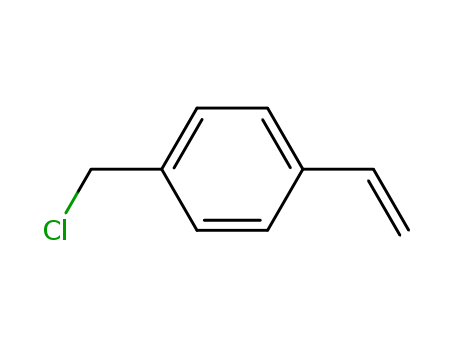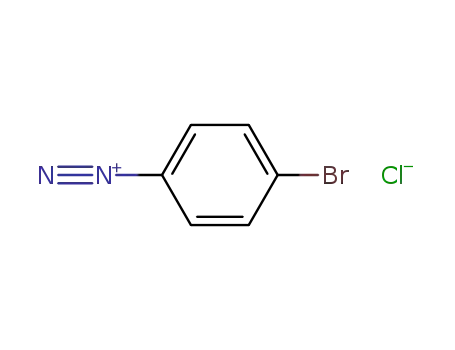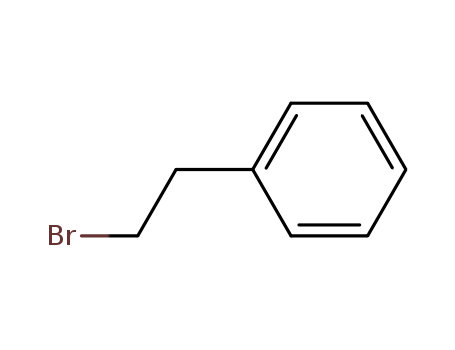Dehydroxymethyl Bromination of Alkoxybenzyl Alcohols by Using a Hypervalent Iodine Reagent and Lithium Bromide
Shibata, Ayako,Kitamoto, Sara,Fujimura, Kazuma,Hirose, Yuuka,Hamamoto, Hiromi,Nakamura, Akira,Miki, Yasuyoshi,Maegawa, Tomohiro
, p. 2275 - 2278 (2018)
We describe the dehydroxymethylbrominati...
Copper-catalyzed halogenation of arylboronic acids
Zhang, Guangyou,Lv, Guanglei,Li, Liping,Chen, Fan,Cheng, Jiang
, p. 1993 - 1995 (2011)
In this Letter, a copper-catalyzed halog...
Gold-catalyzed halogenation of aromatics by N-halosuccinimides
Mo, Fanyang,Yan, Jerry Mingtao,Qiu, Di,Li, Fei,Zhang, Yan,Wang, Jianbo
, p. 2028 - 2032 (2010)
(Chemical Equation Presented) Golden bro...
Site-specific Bromination of Aromatic Compounds: a Rapid Method for Radiobromine Labelling
Adam, Michael J.,Ruth, Thomas J.,Pate, Brian D.,Hall, Laurance D.
, p. 625 - 626 (1982)
Bromobenzene and -p-bromoanisole have be...
Applications of Selenonium Cations as Lewis Acids in Organocatalytic Reactions
He, Xinxin,Wang, Xinyan,Tse, Ying-Lung (Steve),Ke, Zhihai,Yeung, Ying-Yeung
, p. 12869 - 12873 (2018)
The use of trisubstituted selenonium sal...
Electrophilic cleavage reactions of carbon-silicon bonds in neutral hexacoordinate silicon compounds: diorgano(phtalocyaninato)silicon
Tamao, Kohei,Akita, Munetaka,Kato, Hidehito,Kumada, Makoto
, p. 165 - 180 (1988)
Preparations of diorgano(phtalocyaninato...
-
Uemura et al.
, p. 147 (1974)
-
Generation of interhalogen fluorides under mild conditions: A comparison of sluggish and reactive interhalogen fluorides
Shellhamer, Dale F.,Horney, Mark J.,Pettus, Benjamin J.,Pettus, Tobiah L.,Stringer, Joy Merry,Heasley, Victor L.,Syvret, Robert G.,Dobrolsky Jr., John M.
, p. 1094 - 1098 (1999)
Interhalogen fluorides (XF; X = I, Br, o...
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ
Baik, Woonphil,Luan, Wanqiang,Lee, Hyun Joo,Yoon, Cheol Hun,Koo, Sangho,Kim, Byeong Hyo
, p. 213 - 219 (2005)
Halodimethylsulfonium halide 1, which is...
Sodium nitrite-catalyzed oxybromination of aromatic compounds and aryl ketones with a combination of hydrobromic acid and molecular oxygen under mild conditions
Zhang, Guofu,Liu, Renhua,Xu, Qing,Ma, Lixin,Liang, Xinmiao
, p. 862 - 866 (2006)
A novel and efficient catalytic system f...
An efficient, rapid and regioselective nuclear bromination of aromatics and heteroaromatics with NBS using sulfonic-acid-functionalized silica as a heterogeneous recyclable catalyst
Das, Biswanath,Venkateswarlu, Katta,Krishnaiah, Maddeboina,Holla, Harish
, p. 8693 - 8697 (2006)
A simple, efficient and rapid method has...
107. Substitution electrophile aromatique dans l'anhydride sulfureux liquide. Etude cinetique de la reaction de bromination d'anisoles monosubstitues. Transmission des effets electroniques et caracteristiques de l'etat de transition
Castellonese, Paul,Villa, Pierre
, p. 1068 - 1077 (1983)
Reactivity-structure correlations for an...
Solvent effect in the free-radical oxidation and electrophilic ipso and hydrogen displacement of p-methoxybenzyl alcohol and N-(p-methoxybenzyl)acetamide by Br2
Bravo, Anna,Fontana, Francesca,Dordi, Barbara,Minisci, Francesco
, p. 3880 - 3881 (2000)
-
Site directed nuclear bromination of aromatic compounds by an electrochemical method
Raju,Kulangiappar,Anbu Kulandainathan,Uma,Malini,Muthukumaran
, p. 4581 - 4584 (2006)
Direct bromination of a wide range of ar...
Green halogenation reactions for (hetero)aromatic ring systems in alcohol, water, or no solvent
Kajorinne, Jessie K.,Steers, Jennifer C.M.,Merchant, Marnie E.,MacKinnon, Craig D.
, p. 1087 - 1091 (2018)
A new method of brominating aromatic and...
Fast and easy halide exchange in aryl halides
Arvela, Riina K.,Leadbeater, Nicholas E.
, p. 1145 - 1148 (2003)
We report here the rapid halide exchange...
Catalytic Sandmeyer bromination
Beletskaya, Irina P.,Sigeev, Alexander S.,Peregudov, Alexander S.,Petrovskii, Pavel V.
, p. 2534 - 2538 (2007)
An efficient catalyst system for Sandmey...
A new recoverable Au(III) catalyst supported on magnetic polymer nanocomposite for aromatic bromination
Li, Bai,Gao, Linfeng,Bian, Fengling,Yu, Wei
, p. 1063 - 1066 (2013)
This Letter presents a facile alternativ...
Bis(sym-collidine)bromine(I) hexafluorophosphate as oxidant
Rousseau,Robin
, p. 8881 - 8885 (2000)
Primary and secondary alcohols in soluti...
Predicting the structure of supramolecular dendrimers via the analysis of libraries of AB3 and constitutional isomeric AB2 biphenylpropyl ether self-assembling dendrons
Rosen, Brad M.,Wilson, Daniela A.,Wilson, Christopher J.,Peterca, Mihai,Won, Betty C.,Huang, Chenghong,Lipski, Linda R.,Zeng, Xiangbing,Ungar, Goran,Heiney, Paul A.,Percec, Virgil
, p. 17500 - 17521 (2009)
The synthesis of 4′-hydroxy-4-biphenylpr...
One-pot synthesis of 4-aryl-2-aminothiazoles from styrenes and thioureas promoted by tribromoisocyanuric acid
de Andrade, Vitor S.C.,de Mattos, Marcio C.S.
, (2020)
A simple and efficient one-pot protocol ...
In situ acidic carbon dioxide/water system for selective oxybromination of electron-rich aromatics catalyzed by copper bromide
Liu, An-Hua,Ma, Ran,Zhang, Meng,He, Liang-Nian
, p. 38 - 43 (2012)
Carbon dioxide, being one of the major g...
Brominations with Pr4NBr9 as a solid reagent with high reactivity and selectivity
Beck, Thorsten M.,Haller, Heike,Streuff, Jan,Riedel, Sebastian
, p. 740 - 747 (2014)
Tetrapropylammonium nonabromide (Pr4NBr9...
-
Beringer,Mausner
, p. 4535 (1958)
-
Aromatic Halogenation Using N-Halosuccinimide and PhSSiMe3 or PhSSPh
Hirose, Yuuka,Yamazaki, Mirai,Nogata, Misa,Nakamura, Akira,Maegawa, Tomohiro
, p. 7405 - 7410 (2019)
We developed a mild aromatic halogenatio...
-
Forrest,J.
, p. 589 - 592 (1960)
-
Iodine(I) reagents in hydrochloric acid-catalyzed oxidative iodination of aromatic compounds by hydrogen peroxide and iodine
Bedrac, Leon,Iskra, Jernej
, p. 1243 - 1248 (2013)
Hydrochloric acid activates the oxidativ...
ELECTROPHILIC BROMINATION OF PHENOL ETHERS IN SUPERACID SOLUTION USING ALKALI BROMIDE
Cherry, Ghassan,Culmann, Jean-Christophe,Sommer, Jean
, p. 2007 - 2010 (1990)
Electrophilic aromatic bromination of ph...
Selective halogenation of aromatics by dimethyldioxirane and halogen ions
Bovicelli,Mincione,Antonioletti,Bernini,Colombari
, p. 2955 - 2963 (2001)
The oxidation of halogen anions by dimet...
Vanadium-catalysed oxidative bromination using dilute mineral acids and hydrogen peroxide: An option for recycling waste acid streams
Rothenberg, Gadi,Clark, James H.
, p. 270 - 274 (2000)
Vanadium pentoxide, V2O5, catalyses the ...
Direct halogenation of organic compounds with halides using oxone in water - A green protocol
Firouzabadi,Iranpoor,Kazemi
, p. 1675 - 1681 (2009)
Direct bromination and iodination of var...
-
Korzeniowski,Gokel
, p. 3519 (1977)
-
RADICAL DECARBOXYLATIVE BROMINATION OF AROMATIC ACIDS
Barton, Derek H.R.,Lacher, Brigitte,Zard, Samir Z.
, p. 5939 - 5942 (1985)
Thiohydroxamic esters (mixed anhydrides)...
(Diacetoxyiodo)benzene-Lithium Bromide as a Convenient Electrophilic Br+ Source
Braddock, D. Christopher,Cansell, Gemma,Hermitage, Stephen A.
, p. 461 - 464 (2004)
A mild and versatile procedure for the b...
Oxidative bromination reaction using vanadium catalyst and aluminum halide under molecular oxygen
Kikushima, Kotaro,Moriuchi, Toshiyuki,Hirao, Toshikazu
, p. 340 - 342 (2010)
The vanadium-catalyzed oxidative bromina...
A Mild Selective Monobromination Reagent System for Alkoxybenzenes; N-Bromosuccinimide-Silica Gel
Konishi, Hisatoshi,Aritomi, Katsutomo,Okano, Tamon,Kiji, Jitsuo
, p. 591 - 593 (1989)
The monobromination of alkoxybenzenes wi...
An efficient regioselective NBS aromatic bromination in the presence of an ionic liquid
Pingali, Subramanya R.K.,Madhav, Monika,Jursic, Branko S.
, p. 1383 - 1385 (2010)
A simple, efficient, and rapid method wa...
The oxidative halogenations of arenes in water using hydrogen peroxide and halide salts over an ionic catalyst containing sulfo group and hexafluorotitanate
Wang, Ling,Wang, Sa-Sa,Vo-Thanh, Giang,Liu, Ye
, p. 56 - 62 (2013)
An ionic compound, bis[1-methyl-3-(3′-su...
An efficient vanadium-catalyzed bromination reaction
Moriuchi, Toshiyuki,Yamaguchi, Mitsuaki,Kikushima, Kotaro,Hirao, Toshikazu
, p. 2667 - 2670 (2007)
An efficient catalytic oxidative bromina...
Highly efficient halogenation of organic compounds with halides catalyzed by cerium(III) chloride heptahydrate using hydrogen peroxide as the terminal oxidant in water
Firouzabadi, Habib,Iranpoor, Nasser,Kazemi, Somayeh,Ghaderi, Arash,Garzan, Atefeh
, p. 1925 - 1932 (2009)
In this article a new environmentally fr...
Metal- and base-free synthesis of aryl bromides from arylhydrazines
Phuc Tran, Dat,Nomoto, Akihiro,Mita, Soichiro,Dong, Chun-ping,Kodama, Shintaro,Mizuno, Takumi,Ogawa, Akiya
, (2020)
An efficient method was developed to syn...
Poly-N-bromosulfonamide-melamine as a novel brominating reagent for regioselective ipso-bromination of arylboronic acids
Alavinia, Sedigheh,Ghorbani-Vaghei, Ramin
, p. 1269 - 1276 (2021)
A practical synthetic method for the syn...
An intriguing effect of lithium perchlorate dispersed on silica gel in the bromination of aromatic compounds by N-bromosuccinimide
Bagheri, Mojtaba,Azizi, Najmedin,Saidi, Mohammad R.
, p. 146 - 149 (2005)
A convenient and efficient procedure for...
Metal and H2O2 free aerobic oxidative aromatic halogenation with [RNH3+] [NO3-]/HX and [BMIM(SO3H)][NO3)x(X)y] (X = Br, Cl) as multifunctional ionic liquids
Prebil, Rok,Laali, Kenneth K.,Stavber, Stojan
, p. 2108 - 2111 (2013)
Novel multifunctional ionic liquids (ILs...
The X-ray crystal structures and computational analysis of NH...π hydrogen bonded banana-shaped carbazole derivatives and thermal analysis of higher mesogenic homologues
Belloni, Maura,Manickam,Ashton, Peter R.,Kariuki, Benson M.,Preece, Jon A.,Spencer, Neil,Wilkie, John
, p. 17 - 35 (2001)
The synthesis of a series of banana-shap...
Arenediazonium o-benzenedisulfonimides: Some kinetics of azo coupling reactions with naphthols
Boga, Carla,Degani, Jacopo,Del Vecchio, Erminia,Fochi, Rita,Forlani, Luciano,Todesco, Paolo E.
, p. 3837 - 3843 (2002)
Kinetic investigation of azo coupling re...
Anodically-Generated Br-Cl Composite Halogenating Reagents
Fukui, Kouta,Nonaka, Tsutomu
, p. 943 - 948 (1992)
The halogenating power of Br-Cl composit...
Detection of Aryl Radicals in Hydrodediazoniations
Wassmundt, Frederick W.,Kiesman, William F.
, p. 8304 - 8308 (1997)
Iodoacetic acid, an effective aryl radic...
Methanolysis of 4-bromobenzenediazonium ions. Effects of acidity, [MeOH] and temperature on the formation and decomposition of diazo ethers that initiate homolytic dediazoniation
Fernandez-Alonso, Alejandra,Bravo-Diaz, Carlos
, p. 4004 - 4011 (2008)
We have investigated the effects of solv...
Silver catalyzed bromination of aromatics with N-bromosuccinimide
Zhang, Rui,Huang, Lei,Zhang, Yanfang,Chen, Xiaorong,Xing, Weihong,Huang, Jun
, p. 378 - 383 (2012)
A heterogeneous silver catalyst was prep...
AUTOMATED DIAZOMETHANE GENERATOR, REACTOR AND SOLID PHASE QUENCHER
-
Page/Page column 17-18, (2022/03/09)
An automated apparatus (Diazo-M-pen and ...
Eco-Friendly Methodology for the Formation of Aromatic Carbon–Heteroatom Bonds by Using Green Ionic Liquids
Richards, Kenza,Petit, Eddy,Legrand, Yves-Marie,Grison, Claude
supporting information, p. 809 - 814 (2020/11/30)
A new sustainable method is reported for...
Visible-Light Promoted C–O Bond Formation with an Integrated Carbon Nitride–Nickel Heterogeneous Photocatalyst
Vijeta, Arjun,Casadevall, Carla,Roy, Souvik,Reisner, Erwin
supporting information, p. 8494 - 8499 (2021/03/08)
Ni-deposited mesoporous graphitic carbon...
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego