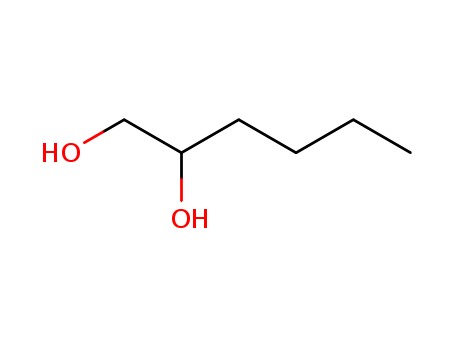
Factory Sells Best Quality Hexane-1,2-diol 6920-22-5 with stock
- Molecular Formula:C6H14O2
- Molecular Weight:118.176
- Appearance/Colour:clear colorless to light yellow liquid
- Vapor Pressure:0.0194mmHg at 25°C
- Melting Point:45oC
- Refractive Index:n20/D 1.442(lit.)
- Boiling Point:223.499 °C at 760 mmHg
- PKA:14.49±0.20(Predicted)
- Flash Point:95.818 °C
- PSA:40.46000
- Density:0.961 g/cm3
- LogP:0.52980
DL-1,2-Hexanediol(Cas 6920-22-5) Usage
|
Preparation
|
1,2-Hexanediol was prepared in about 45% over-all yield by a-bromination of caproic acid, hydrolysis to a-hydroxycaproic acid, and reduction with lithium aluminum hydride.Using the oxidant of H2O2, the organic acid is oxidized to peroxyacid, and the peroxyacid then epoxidizes the olefin double bond, and finally hydrolyzes to obtain 1,2-hexanediol. |
|
benefits
|
1,2 hexanediol is most commonly used as a solvent in skincare formulation. It pulls the moisture up from the deeper levels of the skin, as well as from the air, to help keep the top layers of your skin from drying out. This makes It very effective at keeping your skin hydrated and providing long-term moisture. It can also help to disperse pigments more evenly in makeup products and boost the antimicrobial activity of preservatives. |
|
Flammability and Explosibility
|
Nonflammable |
|
Safety
|
1,2 hexanediol has been proven to be a completely safe and non-irritating ingredient. In an in-use safety evaluation for skin irritation and sensitization potential, 28 participants (males and females) were instructed to use a body wash containing 0.15% 1,2- hexanediol for a minimum of 3 times per week over a 30-day period. There was no evidence of erythema, edema, or dryness of application sites in any of the participants, and it was concluded that the product did not demonstrate a potential for eliciting skin irritation or sensitization.? |
|
Purification Methods
|
Fractionally distil it, preferably in a vacuum. Alternatively, dissolve it in Et2O, dry with K2CO3 then Na2SO4, filter, evaporate and distil it in a vacuum. The bis-4-nitrobenzoyl derivative has m 101.5-102.5o. [Rudloff Can J Chem 36 486 1958, Beilstein 1 I 251, 1 III 2200, 1 IV 2554.] |
|
Definition
|
1,2-Hexanediol is a linear aliphatic diol with a carbon chain length containing six carbons. It is a synthetic preservative and moisture-binding agent belonging to a class of agents known as higher molecular glycols. It is considered non-sensitizing. It is ideal for use as an emollient, humectant, and wetting agent in cosmetic and skin care products. |
|
General Description
|
1,2-Hexanediol acts as cosurfactant, used for modifying the sodium dodecyl sulfate micelles. Solubility of 1,2-hexanediol in supercritical CO2 has been reported. It has a tendency of self-association to form micelle-like aggregates. |
InChI:InChI=1/C6H14O2/c1-2-3-4-6(8)5-7/h6-8H,2-5H2,1H3/t6-/m1/s1
6920-22-5 Relevant articles
Chromium-Catalyzed Production of Diols From Olefins
-
Paragraph 0111, (2021/03/19)
Processes for converting an olefin react...
A METHOD FOR PREPARING 1,2-HEXANEDIOL
-
Paragraph 0040; 0064-0071; 0076, (2021/10/27)
The present invention provides a colourl...
Sterically controlling 2-carboxylated imidazolium salts for one-step efficient hydration of epoxides into 1,2-diols
Cheng, Weiguo,Dong, Li,Fu, Mengqian,Su, Qian,Tan, Xin,Yao, Xiaoqian,Ying, Ting,Zhang, Suojiang
, p. 2992 - 3000 (2021/05/07)
In order to overcome the disadvantages o...
Diol-Ritter Reaction: Regio- And Stereoselective Synthesis of Protected Vicinal Aminoalcohols and Mechanistic Aspects of Diol Monoester Disproportionation
Abboud, Khalil A.,Cheng, Kevin,Klosin, Jerzy,Kruper, William J.,Kruper, William R.,Lysenko, Ivan,Ondari, Mark E.,Thomas, Pulikkottil J.
, (2021/10/20)
The well-known epoxide-Ritter reaction g...
6920-22-5 Process route
-
-
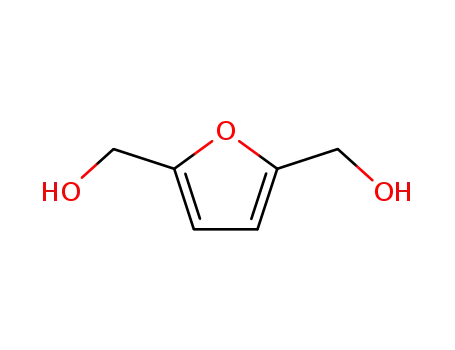
1883-75-6
2,5-bis-(hydroxymethyl)furan
-
-
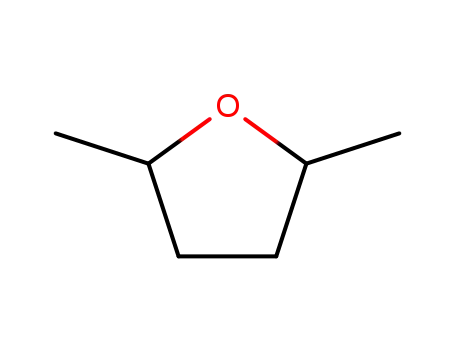
1003-38-9
2,5-dimethyltetrahydrofuran
-
-
625-86-5
2,5-dimethylfuran
-
-
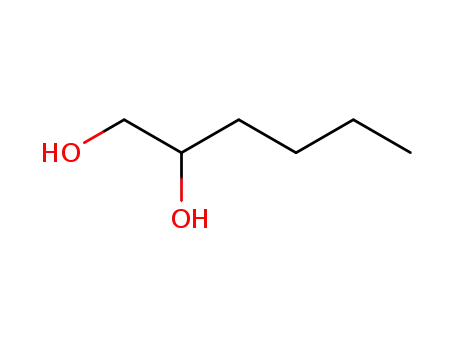
6920-22-5
Hexane-1,2-diol
-
-
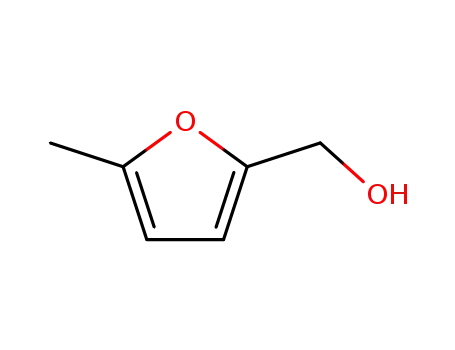
3857-25-8
2-hydroxymethyl-5-methylfuran
Conditions
| Conditions |
Yield |
|
With
hydrogen;
In
ethanol;
at 220 ℃;
for 3h;
under 37503.8 Torr;
|
|
-
-
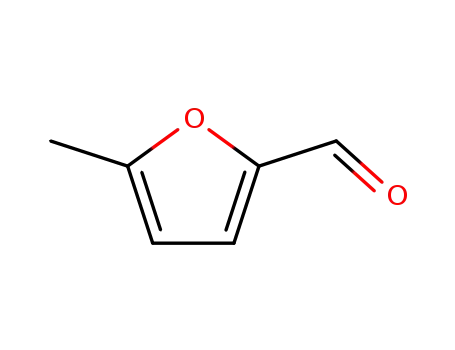
620-02-0
5-Methylfurfural
-
-
1003-38-9
2,5-dimethyltetrahydrofuran
-
-
625-86-5
2,5-dimethylfuran
-
-
6920-22-5
Hexane-1,2-diol
-
-
3857-25-8
2-hydroxymethyl-5-methylfuran
Conditions
| Conditions |
Yield |
|
With
hydrogen;
In
butan-1-ol;
at 199.84 ℃;
for 3h;
under 22502.3 Torr;
Autoclave;
|
|
6920-22-5 Upstream products
-
1436-34-6
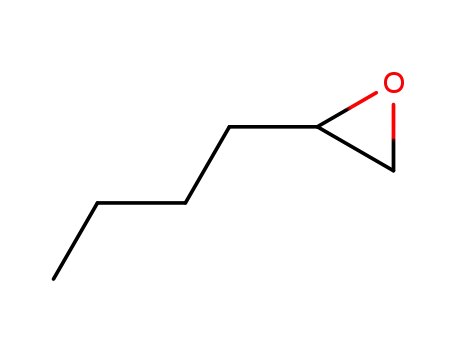
1,2-Epoxyhexane
-
93339-59-4
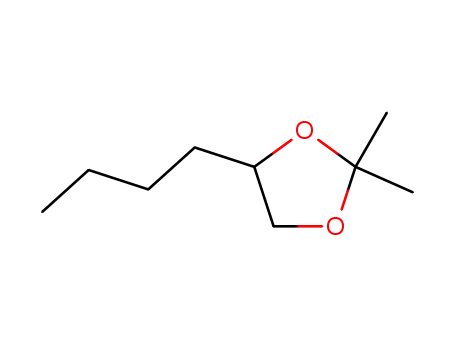
4-butyl-2,2-dimethyl-[1,3]dioxolane
-
592-41-6
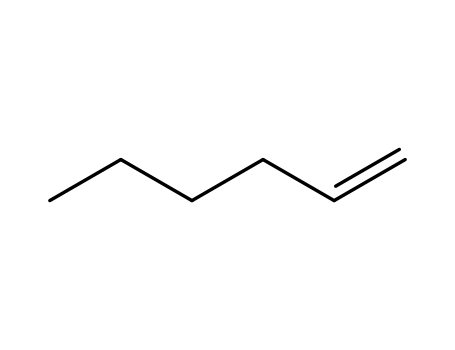
1-hexene
-
636-36-2
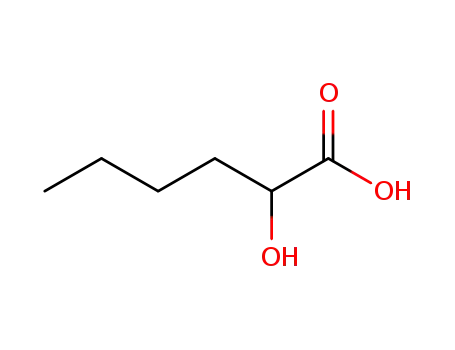
rac-2-hydroxyhexanoic acid
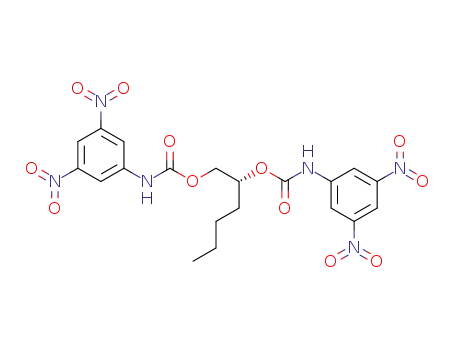
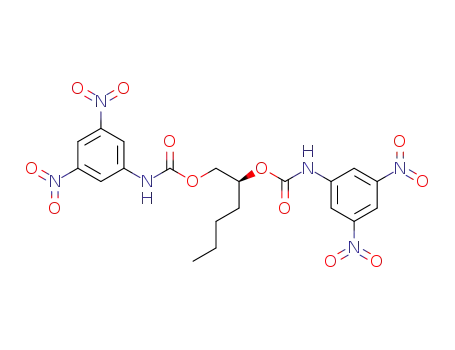
6920-22-5 Downstream products
-
128126-11-4
C20H20N6O12
-
128126-11-4
C20H20N6O12
-
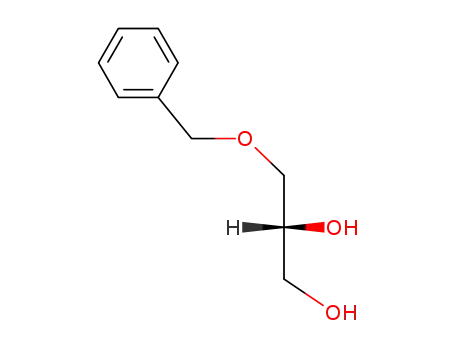
143508-41-2
Z-Glu<(2S,2R)-2-hydroxyhexyl>-OtBu
-
56552-80-8
(R)-3-benzyloxy-1,2-propanediol
 English
English 中文
中文
 English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego
English
Japanese
Russian
Korean
गोंगेन हें नांव
Deutsch
Corsu
Guarani
Hausa
Cymraeg
Nederlands
Aymara
Français
Kreyòl ayisyen
čeština
ʻŌlelo Hawaiʻi
डोग्रिड ने दी
ภาษาไทย
հայերեն
فارسی
Hmoob
ދިވެހި
भोजपुरी
繁體中文
Türkçe
हिंदी
беларускі
български
tur
Gaeilge
ગુજરાતી
Magyar
Eesti keel
بالعربية
বাংলা
Azərbaycan
Português
Suid-Afrikaanse Dutch taal
کوردی-سۆرانی
Ελληνικά
español
Frysk
dansk
አማርኛ
Bamanankan
euskara
Italiano
Tiếng Việt
অসমীয়া
català
Suomalainen
Eʋegbe
Hrvatski
Cebuano
Gàidhlig na h-Alba
bosanski
galego